Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagai, Takayuki; Okamoto, Yoshihiro; Shibata, Daisuke*; Kojima, Kazuo*; Hasegawa, Takehiko*; Sato, Seiichi*; Fukaya, Akane*; Hatakeyama, Kiyoshi*
JAEA-Research 2024-014, 54 Pages, 2025/02
XAFS measurements in the soft X-ray region are suitable for evaluating the chemical state of the surface layer of a measurement sample because the X-ray transmittance is low. In this study, the purpose of the study was to confirm the difference between the coagulated surface layer and the inside of the simulated waste glasses by measuring the K-edge of the glass constituent elements boron, oxygen, sodium, and silicon, and the L edge of the waste component cerium. As a result, the B K-edge XANES spectra showed that the proportion of B-O tetracoordinate sp
edge of the waste component cerium. As a result, the B K-edge XANES spectra showed that the proportion of B-O tetracoordinate sp structures (BO
structures (BO ) on the surface layer of the coagulated glass samples was higher than that on the cut surface inside the glass samples, which is expected to improve the water resistance of the coagulated surface. On the other hand, the O K-edge XANES spectra suggested that the O abundance in the coagulated surface layer was lower than that in the cut surface inside the glass samples, and that alkali metal elements may be concentrated in the coagulated surface layer. However, no difference was observed in the Na K-edge XANES spectra between the coagulated surface layer and the cut surface, and no difference was observed in the Si K-edge XANES spectra between the solidified surface and the inside of glass samples. In addition, the Ce L
) on the surface layer of the coagulated glass samples was higher than that on the cut surface inside the glass samples, which is expected to improve the water resistance of the coagulated surface. On the other hand, the O K-edge XANES spectra suggested that the O abundance in the coagulated surface layer was lower than that in the cut surface inside the glass samples, and that alkali metal elements may be concentrated in the coagulated surface layer. However, no difference was observed in the Na K-edge XANES spectra between the coagulated surface layer and the cut surface, and no difference was observed in the Si K-edge XANES spectra between the solidified surface and the inside of glass samples. In addition, the Ce L -edge XANES spectra confirmed that the Ce valence in the surface layer of coagulated glass samples were oxidized compared to the inside of glass samples.
-edge XANES spectra confirmed that the Ce valence in the surface layer of coagulated glass samples were oxidized compared to the inside of glass samples.
Sato, Nobuaki*; Kameo, Yutaka; Sato, Soichi; Kumagai, Yuta; Sato, Tomonori; Yamamoto, Masahiro*; Watanabe, Yutaka*; Nagai, Takayuki; Niibori, Yuichi*; Watanabe, Masayuki; et al.
Introduction to Dismantling and Decommissioning Chemistry, 251 Pages, 2024/09
This book focuses on the dismantling and decommissioning of nuclear facilities and reactors that have suffered severe accidents. In Part 1, we introduce basic aspects ranging from fuel chemistry, analytical chemistry, radiation chemistry, corrosion, and decontamination chemistry to waste treatment and disposal. Then, Part 2 covers the chemistry involved in the decommissioning of various nuclear facilities, and discusses what chemical approaches are necessary and possible for the decommissioning of TEPCO's Fukushima Daiichi Nuclear Power Plants, how decommissioning should be carried out, and what kind of research and development and also human resource development are required for this.
 Ba
Ba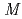 (PO
(PO )
) (
(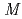 = Mg, Mn, Co, and Ni)
= Mg, Mn, Co, and Ni)Kajita, Yoichi*; Nagai, Takayuki*; Yamagishi, Shigetada*; Kimura, Kenta*; Hagihara, Masato; Kimura, Tsuyoshi*
Chemistry of Materials, 36(15), p.7451 - 7458, 2024/08
Times Cited Count:2 Percentile:51.02(Chemistry, Physical)Nagai, Takayuki*; Hagihara, Masato; Yokoi, Rie*; Moriwake, Hiroki*; Kimura, Tsuyoshi*
Journal of the American Chemical Society, 146(33), p.23348 - 23355, 2024/08
Times Cited Count:1 Percentile:31.48(Chemistry, Multidisciplinary)Katsuoka, Nanako; Akiyama, Daisuke*; Kirishima, Akira*; Nagai, Takayuki; Okamoto, Yoshihiro; Sato, Nobuaki*
2023-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2024/07
no abstracts in English
Nagai, Takayuki; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2023 (Internet), 3 Pages, 2024/00
no abstracts in English
Nagai, Takayuki; Hasegawa, Takehiko*
JAEA-Research 2023-008, 41 Pages, 2023/12
To reduce the risks posed by stored the high-level radioactive liquid waste (HAW), Tokai Vitrification Facility (TVF) is working to produce the HAW into vitrified bodies. With the aim of steady vitrification of HAW in TVF, the vitrification technology section has manufactured a new 3rd melter with an improved bottom structure and is working to verify the performance of this melter. In this study, solidified glass samples were taken from simulated vitrified bodies produced by flowing molten glass during the bottom drain-out test in the operation confirmation of the TVF 3rd melter. And the properties of the surface layer and fracture surface of the vitrified bodies were evaluated by using Raman spectroscopy, synchrotron radiation XAFS measurement, and laser ablation inductively coupled plasma atomic emission spectroscopy (LA ICP-AES) analysis. As a result of measuring the surface layer and fracture surface of the solidified samples produced on an actual scale, a slight difference was confirmed between the properties of the surface layer and those of the fracture surface. Since the chemical composition of these simulated vitrified bodies does not contain platinum group elements, it is expected that the glass structure of solidified glass samples is different from that of the actual vitrified body. However, this sample measuring was a valuable opportunity to evaluate samples produced by using the direct energized joule heating method. The properties of cullet used the operation confirmation of the TVF 3rd melter and the cullet of another production lot were measured and analyzed in the same manner under the measuring conditions of solidified glass samples. As a result, it was confirmed that cullet with different producing histories have different glass structures even with the same chemical composition, and that differences in glass structures remain in the glass samples after melting these cullet.
Nagai, Takayuki; Okamoto, Yoshihiro; Yamagishi, Hirona*; Shibata, Daisuke*; Kojima, Kazuo*; Hasegawa, Takehiko*; Sato, Seiichi*; Fukaya, Akane*; Hatakeyama, Kiyoshi*
JAEA-Research 2023-004, 45 Pages, 2023/09
The local structure of glass-forming elements and waste elements in borosilicate glasses varies with its chemical composition. In this study, simulated waste glass samples were prepared and the chemical state regarding boron (B), silicon (Si) and waste elements of iron (Fe), cesium (Cs) were estimated by using XAFS measurement in soft X-ray region. To understand the chemical stability of simulated waste glasses, XANES spectra of B K-edge, Fe L , L
, L -edge, and Cs M
-edge, and Cs M , M
, M -edge were measured on the glass surface exposed to the leachate. As a result, it was found that the glass surface exposed to the leachate was changed and it was difficult to obtain a clear XANES spectrum. From the B K-edge XANES spectra on glass surfaces exposed to the leachate, an increase in three-coordination of B-O (BO
-edge were measured on the glass surface exposed to the leachate. As a result, it was found that the glass surface exposed to the leachate was changed and it was difficult to obtain a clear XANES spectrum. From the B K-edge XANES spectra on glass surfaces exposed to the leachate, an increase in three-coordination of B-O (BO ) and a decrease in four-coordination of B-O (BO
) and a decrease in four-coordination of B-O (BO ) were observed compared to the glass surfaces before immersion. The XANES spectra of Fe L
) were observed compared to the glass surfaces before immersion. The XANES spectra of Fe L , L
, L -edge, and Cs M
-edge, and Cs M , M
, M -edge show that as the exposure time in the leachate increases, the Cs present on the glass surface dissolves into the leachate. The XANES spectra of Si K-edge were measured on simulated waste glass surfaces before immersion, and it was confirmed that the change in XANES spectra given by Na
-edge show that as the exposure time in the leachate increases, the Cs present on the glass surface dissolves into the leachate. The XANES spectra of Si K-edge were measured on simulated waste glass surfaces before immersion, and it was confirmed that the change in XANES spectra given by Na O concentration had a larger effect than the waste component concentration.
O concentration had a larger effect than the waste component concentration.
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Katsuoka, Nanako; Okamoto, Yoshihiro; Sato, Nobuaki*
2022-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2023/09
no abstracts in English
Oba, Yojiro; Motokawa, Ryuhei; Kaneko, Koji; Nagai, Takayuki; Tsuchikawa, Yusuke; Shinohara, Takenao; Parker, J. D.*; Okamoto, Yoshihiro
Scientific Reports (Internet), 13, p.10071_1 - 10071_8, 2023/06
Times Cited Count:3 Percentile:59.85(Multidisciplinary Sciences)Nagai, Takayuki
JAEA-Research 2022-014, 84 Pages, 2023/02
Most of the simulated waste glasses used for physical property evaluation are processed into a shape suitable for the measurement method from glass gob obtained by slowly cooling molten glass to room temperature. However, the actual vitrified waste glass material is obtained by cooling and being coagulated the glass drained from the bottom of glass melter into the canister. In this study, Raman spectroscopy was performed on the coagulated surface of molten simulated waste glass in the depth direction to evaluate the state of the Si-O bridging structure near the coagulated glass surface. The Raman spectra measured from the surface to the depth direction near the surface of the glasses produced by several melting and coagulation conditions of molten simulated waste glass cullet in the air atmosphere, and it was confirmed that there were changes in these spectra. On the other hand, the raw material glass cullet and the surface of the glass solidified in argon gas atmosphere showed little change in the spectrum in the depth direction, and the Si-O bridging structure near the glass surface was similar. It was also confirmed that the spectrum change in the depth direction measurement was small for the cut surface of the glass, and that the change in the spectrum for the broken glass fracture surface was also small. For glasses with a large change in Raman spectra in the depth direction near the coagulated surface, the molten glass was cooled from the molten state to room temperature in a muffle furnace with air atmosphere. That is, the magnitude of the spectral change with respect to the depth direction depends on the time from the molten state to coagulation. In order to confirm the reason why the number of bridging oxygen in the Si-O bridging structure is small on the coagulated surface of glass, the XANES spectra of Si-K edge and Ce-L3 edge were measured by XAFS on the coagulated surface and the cutting face. As a result, the Si-K edge peak on the coagulated surface is
Nagai, Takayuki; Tone, Masaya; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2022 (Internet), 3 Pages, 2023/00
no abstracts in English
Okamoto, Yoshihiro; Shiwaku, Hideaki; Shimamura, Keisuke*; Kobayashi, Hidekazu; Nagai, Takayuki; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
Journal of Nuclear Materials, 570, p.153962_1 - 153962_13, 2022/11
Simulated nuclear waste glass samples containing phosphorus, which increase the solubility of molybdenum, were prepared and analyzed using synchrotron X-ray Absorption Fine Structure (XAFS) analysis for some constituent elements and Raman spectroscopic analysis of their complex structure. Changes in local structure and chemical state due to different phosphorus additions and waste loading rates were systematically studied. Consequently, no crystalline phase due to the molybdate compound was observed even at a maximum waste content of 30 wt% (corresponding to 1.87 mol% MoO ). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO
). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO structural unit and may deprive the alkali metal coordinated to the molybdate ion.
structural unit and may deprive the alkali metal coordinated to the molybdate ion.
Nagai, Takayuki; Okamoto, Yoshihiro; Yamagishi, Hirona*; Kojima, Kazuo*; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
JAEA-Research 2022-008, 37 Pages, 2022/10
The local structure of glass-forming elements and waste elements in borosilicate glasses varies with its chemical composition. In this study, borosilicate glass frit and simulated waste glass samples were prepared and the chemical state regarding boron (B), silicon (Si) and waste elements of iron (Fe), cesium (Cs) were estimated by using XAFS measurement in soft X-ray region. Following results were obtained by XAFS measurements of simulated waste glass surfaces after immersion test to investigate the long chemical stability. (1) As the leaching time of glass samples in immersion test passed, the Cs M , M
, M -edge XANES spectra disappeared and the Fe L
-edge XANES spectra disappeared and the Fe L , L
, L -edge spectra changed. (2) A new compound was formed on the sample surface after the immersion test, and these changes in the surface state were confirmed by Raman spectroscopy. However, it became difficult to obtain a clear B K-edge XANES spectrum by forming a compound on glass surfaces. The Si K-edge XANES spectra of borosilicate glass frits with different Na
-edge spectra changed. (2) A new compound was formed on the sample surface after the immersion test, and these changes in the surface state were confirmed by Raman spectroscopy. However, it became difficult to obtain a clear B K-edge XANES spectrum by forming a compound on glass surfaces. The Si K-edge XANES spectra of borosilicate glass frits with different Na O content were measured, and following was confirmed. (1) As the Na
O content were measured, and following was confirmed. (1) As the Na O concentration increases in borosilicate glass frit, the K-edge peak of Si shifts to the low energy side. (2) The intensity of the Si K-edge peak is maximum when the Na
O concentration increases in borosilicate glass frit, the K-edge peak of Si shifts to the low energy side. (2) The intensity of the Si K-edge peak is maximum when the Na O content in glass frits was about 7wt%.
O content in glass frits was about 7wt%.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:4 Percentile:51.78(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U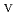
 Fe
Fe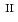
 U
U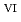 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Motokawa, Ryuhei; Kaneko, Koji; Oba, Yojiro; Nagai, Takayuki; Okamoto, Yoshihiro; Kobayashi, Taishi*; Kumada, Takayuki; Heller, W. T.*
Journal of Non-Crystalline Solids, 578, p.121352_1 - 121352_7, 2022/02
Times Cited Count:4 Percentile:19.44(Materials Science, Ceramics)Nagai, Takayuki; Okamoto, Yoshihiro; Yamagishi, Hirona*; Ota, Toshiaki*; Kojima, Kazuo*; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
JAEA-Research 2021-010, 62 Pages, 2022/01
The local structure of glass-forming elements and waste elements in borosilicate glasses varies with its chemical composition. In this study, borosilicate glass frit and simulated waste glass samples were prepared and the local structure and chemical state regarding boron(B), oxygen(O), silicon(Si) and waste elements of iron(Fe), cesium(Cs) were estimated by using XAFS measurement in soft X-ray region. Following results were obtained by XAFS measurements of prepared glass frit and simulated waste glass samples: (1) The effect of Na O concentration on B-O coordination structure is greater than that of the waste elements concentration. (2) The height of pre-edge by O K-edge spectrum depends on the concentration of first transition elements such as Fe in glass samples. Following results were obtained by XAFS measurements of simulated waste glass samples after immersion test to investigate the long chemical stability. (1) A new compound was formed on the sample surface after the immersion test, and changes in the surface state were confirmed by Raman spectroscopy. (2) Cs on the sample surface after immersion test dissolves into the leaching solution. The Si K-edge XANES spectra of borosilicate glass frits and simulated waste glass samples included lanthanides oxide were measured, and following was confirmed. (1) As the Na
O concentration on B-O coordination structure is greater than that of the waste elements concentration. (2) The height of pre-edge by O K-edge spectrum depends on the concentration of first transition elements such as Fe in glass samples. Following results were obtained by XAFS measurements of simulated waste glass samples after immersion test to investigate the long chemical stability. (1) A new compound was formed on the sample surface after the immersion test, and changes in the surface state were confirmed by Raman spectroscopy. (2) Cs on the sample surface after immersion test dissolves into the leaching solution. The Si K-edge XANES spectra of borosilicate glass frits and simulated waste glass samples included lanthanides oxide were measured, and following was confirmed. (1) As the Na O concentration increases in borosilicate glass frit, the K-edge peak of Si shifts to the low energy side. (2) The peak height of the K-edge of Si differs depending on the kind of lanthanide.
O concentration increases in borosilicate glass frit, the K-edge peak of Si shifts to the low energy side. (2) The peak height of the K-edge of Si differs depending on the kind of lanthanide.
Nagai, Takayuki; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*; Arima, Tatsumi*
Photon Factory Activity Report 2021 (Internet), 2 Pages, 2022/00
no abstracts in English
 Ln
Ln O
O (Ln = Gd, Er) solid solution
(Ln = Gd, Er) solid solutionPham, V. M.*; Arima, Tatsumi*; Inagaki, Yaohiro*; Idemitsu, Kazuya*; Akiyama, Daisuke*; Nagai, Takayuki; Okamoto, Yoshihiro
Journal of Nuclear Materials, 556, p.153189_1 - 153189_9, 2021/12
Times Cited Count:1 Percentile:9.64(Materials Science, Multidisciplinary)The crystal structure change was evaluated for (1-x)UO -xLnO
-xLnO (Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H
(Ln=Gd or Er; x = 0 to 0.4) samples sintered at 1973 K for 8 h under Ar and Ar-10%H atmospheres. The effect of LnO
atmospheres. The effect of LnO doping on the crystal structure of UO
doping on the crystal structure of UO was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO
was investigated by X-ray diffraction (XRD) and X-ray absorption fine structure (XAFS). LnO doping into UO
doping into UO reduced the lattice parameter of UO
reduced the lattice parameter of UO -LnO
-LnO solid solutions up to 40mol% LnO
solid solutions up to 40mol% LnO . The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O
. The lattice parameters of these samples were comparable to those of stoichiometric (U,Ln)O solid solutions, that is, the O/M ratios were close to 2.00. The U L
solid solutions, that is, the O/M ratios were close to 2.00. The U L -edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
-edge XANES analysis showed that higher U oxidation states of +5 or +6 formed, in addition to + 4. The EXAFS analysis indicated that the interatomic distances of U-O and Gd-O decreased with increasing x, whereas those of Er-O may not decrease monotonically.
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Okamoto, Yoshihiro
2020-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Oyobi "Hito, Kankyo To Busshitsu O Tsunagu Inobeshion Soshutsu Dainamikku, Araiansu" Kenkyu Seika, Katsudo Hokokusho (CD-ROM), 1 Pages, 2021/11
no abstracts in English