Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
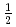 ladder
ladderUeda, Hiroshi*; Onoda, Shigeki*; Yamaguchi, Yasuhiro*; Kimura, Tsuyoshi*; Yoshizawa, Daichi*; Morioka, Toshiaki*; Hagiwara, Masayuki*; Hagihara, Masato*; Soda, Minoru*; Masuda, Takatsugu*; et al.
Physical Review B, 101(14), p.140408_1 - 140408_6, 2020/04
Times Cited Count:6 Percentile:24.90(Materials Science, Multidisciplinary)Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemistry and Biophysics Reports (Internet), 6, p.220 - 225, 2016/07
Matsuo, Tatsuhito; Takeda, Soichi*; Oda, Toshiro*; Fujiwara, Satoru
Biophysics and Physicobiology (Internet), 12, p.145 - 158, 2015/12
Yamaga, Chikanobu; Tomikawa, Hirofumi; Kobayashi, Naoki; Naoi, Yosuke; Oda, Tetsuzo; Mochiji, Toshiro
JAEA-Review 2015-023, 108 Pages, 2015/10
The JAEA held "International Forum on Peaceful Use of Nuclear Energy, Nuclear Non-proliferation and Nuclear Security -Future direction toward promoting non-proliferation and the ideal method of developing human resources using COEs following the New Strategic Energy Plan -" on 3 December 2014. In the Forum, officials and experts from Japan, the United States explained their efforts. Discussion was made in two panels, entitled "Effective and efficient measures to ensure nuclear non-proliferation based on domestic and foreign issues and the direction and role of technology development" and "Roles of nuclear security COEs and future expectations". In Panel Discussion 1, how to implement effective and efficient safeguards was discussed. In Panel Discussion 2, panelists discussed current status of NSSCs and COEs, new role for COEs and regional cooperation. This report includes abstracts of keynote speeches, summaries of two panel discussions and materials of the presentations in the forum.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemical and Biophysical Research Communications, 459(3), p.493 - 497, 2015/04
Times Cited Count:5 Percentile:15.05(Biochemistry & Molecular Biology)Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
Biophysics, 9, p.99 - 106, 2013/07
Fujiwara, Satoru; Plazanet, M.*; Oda, Toshiro*
Biochemical and Biophysical Research Communications, 431(3), p.542 - 546, 2013/02
Times Cited Count:4 Percentile:10.33(Biochemistry & Molecular Biology)Ito, Takuto*; Deki, Manato; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
Proceedings of 12th International Symposium on Laser Precision Microfabrication (LPM 2011) (Internet), 5 Pages, 2011/06
Fujiwara, Satoru; Plazanet, M.*; Matsumoto, Fumiko; Oda, Toshiro*
European Biophysics Journal, 40(5), p.661 - 671, 2011/05
Times Cited Count:11 Percentile:31.40(Biophysics)Deki, Manato; Ito, Takuto*; Yamamoto, Minoru*; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
Applied Physics Letters, 98(13), p.133104_1 - 133104_3, 2011/03
Times Cited Count:13 Percentile:47.65(Physics, Applied)Deki, Manato; Ito, Takuto*; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
Proceedings of 9th International Workshop on Radiation Effects on Semiconductor Devices for Space Applications (RASEDA-9), p.218 - 221, 2010/10
Matsumoto, Fumiko; Deshimaru, Shungo*; Oda, Toshiro*; Fujiwara, Satoru
Analytical Biochemistry, 399(2), p.299 - 301, 2010/04
Times Cited Count:2 Percentile:58.29(Biochemical Research Methods)We have developed a technique by which muscle thin filaments are reconstituted from the recombinant troponin components and the native thin filaments. By this technique, the reconstituted troponin complex is exchanged into the native thin filaments in the presence of 20% glycerol and 0.3 M KCl at pH 6.2. Over 90% of endogenous troponin complex was replaced with the recombinant troponin complex. Structural integrity and Ca -sensitivity of the reconstituted thin filament prepared by this technique was confirmed by X-ray fiber diffraction measurements and the thin filament activated myosin subfragment 1 ATPase measurements, respectively.
-sensitivity of the reconstituted thin filament prepared by this technique was confirmed by X-ray fiber diffraction measurements and the thin filament activated myosin subfragment 1 ATPase measurements, respectively.
Tomita, Takuro*; Iwami, Masahiro*; Yamamoto, Minoru*; Deki, Manato*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Nakagawa, Yoshinori*; Kitada, Takahiro*; Isu, Toshiro*; Saito, Shingo*; et al.
Materials Science Forum, 645-648, p.239 - 242, 2010/04
no abstracts in English
Fujiwara, Satoru; Plazanet, M.*; Matsumoto, Fumiko; Oda, Toshiro*
Biophysical Journal, 94(12), p.4880 - 4889, 2008/06
Times Cited Count:9 Percentile:20.76(Biophysics)Actin plays crucial roles in various aspects of cell motility. Flexibility of F-actin, a filamentous polymer formed by polymerization of the monomers (G-actin), is important for such a variety of functions. This flexibility allows F-actin to interact with various proteins, thereby expressing multiple functions. Understanding the variety of functions of actin thus requires understanding the flexibility of F-actin. As a first step towards this ultimate purpose, we carried out elastic incoherent neutron scattering (EINS) experiments on G-actin and F-actin under hydrated states. The mean square displacement (MSD) was estimated from the EINS measurements. Temperature dependence of MSD showed that two dynamical transitions occur at about 150 K and about 245 K, and that behavior of MSD is different between G-actin and F-actin, such that G-actin is "softer" than F-actin. The different behavior observed is ascribed to the differences in dynamical heterogeneity between F-actin and G-actin.
Deki, Manato; Ito, Takuto*; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
no journal, ,
no abstracts in English
 sensitization in the myofibrils
sensitization in the myofibrilsMatsumoto, Fumiko; Maeda, Kayo*; Nitanai, Yasushi*; Oda, Toshiro*; Maeda, Yuichiro*; Fujiwara, Satoru
no journal, ,
Familial hypertrophic cardiomyopathy (HMC) has been reported to be caused by mutations in a regulatory protein, troponin (Tn). HMC is characterized by functional aberration on the force-pCa relationship. Only a few cardiomyopathy-causing mutations have been mapped on the coiled-coil region (IT-arm) in the Tn core domain. Here we focus on two mutations in the IT-arm, E244D and K247R of TnT. Whereas E244D has been reported to show an increase of the maximum level of ATPase activity, the functional consequence of K247R mutation has not been analyzed. In order to understand how these mutations cause functional aberrations, we measured ATPase activity of myofibrils containing various mutants of these residues (E244; D, M, A, K and K247; R, E, A). The maximum level of ATPase activity was found to increase in K247R, without changing the Ca ion sensitivity, as found in E244D. A close inspection of the crystal structure showed that the side chains of E244 and K247 form the triplet with that of E110 of TnI on the outside of the hydrophobic core of the coiled-coil. This triplet is thus likely to introduce flexibility into the IT-arm at this position. The mutations at the residues 244 and 247 could alter this flexibility. The results obtained here suggested that the proper flexibility of the IT-arm is important for the correct function of the myofibrils. The relationship between the IT-arm flexibility and functional aberration in the myofibril will be discussed.
Deki, Manato; Yamamoto, Minoru*; Ito, Takuto*; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
no journal, ,
no abstracts in English
Ito, Takuto*; Deki, Manato; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
no journal, ,
no abstracts in English
Deki, Manato*; Ito, Takuto*; Yamamoto, Minoru*; Tomita, Takuro*; Matsuo, Shigeki*; Hashimoto, Shuichi*; Kitada, Takahiro*; Isu, Toshiro*; Onoda, Shinobu; Oshima, Takeshi
no journal, ,
no abstracts in English
Fujiwara, Satoru; Plazanet, M.*; Matsumoto, Fumiko; Oda, Toshiro*
no journal, ,
F-actin expresses a variety of functions related to cell motility. To understand how such multiple functions are possible, it is important to understand dynamical properties of F-actin at various levels from internal dynamics of the monomers through relative motions between the monomers to large-scale motions of F-actin. As a first step towards this ultimate purpose, we carried out elastic incoherent neutron scattering (EINS) experiments and quasi-elastic neutron scattering (QENS) experiments of F-actin and G-actin. It was shown from the EINS experiments that there are differences in the internal dynamics of F-actin and G-actin. Analysis of the QENS spectra on F-actin and G-actin showed that both in F-actin and G-actin, there are at least two populations of motions with distinct amplitudes and rates, that higher hydration ratios make these motions "faster", and that G-actin tends to have the motions with larger amplitudes and higher rates than those in F-actin.