Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohashi, Yusuke; Shimaike, Masamitsu; Matsumoto, Takashi; Takahashi, Nobuo; Yokoyama, Kaoru; Morimoto, Yasuyuki
Nuclear Technology, 209(5), p.777 - 786, 2023/05
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)At the Ningyo-Toge Center, technical development related to uranium refining conversion and enrichment has been completed, and decommissioning of these facilities has begun. The error between the quantity of dismantled materials estimated from the facility design drawings and the actual quantity of dismantled materials was minimal when averaging over the entire Uranium Refining and Conversion Plant and Uranium Enrichment Engineering Facility, which results indicated that the preliminary estimate of the quantity of dismantled materials for decommissioning was reasonable. Most of the dismantled materials, which have no contamination history and are properly managed were able to be carried out to recyclers as non-radioactive waste (NR). In addition, the possibility of evaluating the uranium concentration of clearance level in dismantled objects was confirmed through gamma-ray measurement tests using mock-up waste.
Yokoyama, Kaoru; Ohashi, Yusuke
Annals of Nuclear Energy, 175, p.109240_1 - 109240_7, 2022/09
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Decommissioning is planned at nuclear facilities that have been discontinued. We examined the evaluation method of uranium radioactivity for concrete waste generated by the decommissioning of nuclear facilities. Since the peaks of Ac-228, Tl-208, and K- 40 are derived from concrete waste, it is difficult to distinguish the 1001 keV peak emitted from the uranium source. We have derived a formula to correct gamma rays from concrete and the environment, and the amount of uranium was quantified. When the weight of concrete waste is about 300 kg, if the weight of uranium is 3 g or more, it can be quantified within a relative error of about 30%. Measurement tests were performed using homogeneous simulated concrete waste. Since uranium contamination is on the concrete surface at the uranium processing facility and small chunks generated by scraping the concrete surface will be stored in a drum and measured, it seems that the test of homogeneous concrete reflects the actual waste.
Ohashi, Yusuke
Nuclear Instruments and Methods in Physics Research A, 1025, p.166128_1 - 166128_8, 2022/02
Times Cited Count:0 Percentile:0.02(Instruments & Instrumentation)Much metal waste is generated during decommissioning of uranium processing facilities. In this paper, measurement tests were performed on a typical simulated metal waste generated by the decommissioning using the clearance measurement device equipped with NaI detectors, and the results were compared with the simulation results by the MCNP code. Results showed the proper placement of dismantled materials in a drum can be estimated by evaluating the detection efficiency of each waste placement by simulation in advance. Moreover, it was confirmed that the relative errors between simulation and measurement results were within 35% at 0.1 Bq/g. It was found that the uranium concentration below the clearance level (1 Bq/g) can be confirmed even if the error is included, and the clearance level can be safely verified by considering the relative error between the simulation result and the experimental result.
Takeda, Tetsuaki*; Inagaki, Yoshiyuki; Aihara, Jun; Aoki, Takeshi; Fujiwara, Yusuke; Fukaya, Yuji; Goto, Minoru; Ho, H. Q.; Iigaki, Kazuhiko; Imai, Yoshiyuki; et al.
High Temperature Gas-Cooled Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.5, 464 Pages, 2021/02
As a general overview of the research and development of a High Temperature Gas-cooled Reactor (HTGR) in JAEA, this book describes the achievements by the High Temperature Engineering Test Reactor (HTTR) on the designs, key component technologies such as fuel, reactor internals, high temperature components, etc., and operational experience such as rise-to-power tests, high temperature operation at 950 C, safety demonstration tests, etc. In addition, based on the knowledge of the HTTR, the development of designs and component technologies such as high performance fuel, helium gas turbine and hydrogen production by IS process for commercial HTGRs are described. These results are very useful for the future development of HTGRs. This book is published as one of a series of technical books on fossil fuel and nuclear energy systems by the Power Energy Systems Division of the Japan Society of Mechanical Engineers.
C, safety demonstration tests, etc. In addition, based on the knowledge of the HTTR, the development of designs and component technologies such as high performance fuel, helium gas turbine and hydrogen production by IS process for commercial HTGRs are described. These results are very useful for the future development of HTGRs. This book is published as one of a series of technical books on fossil fuel and nuclear energy systems by the Power Energy Systems Division of the Japan Society of Mechanical Engineers.
Yokoyama, Kaoru; Ohashi, Yusuke
Annals of Nuclear Energy, 141, p.107299_1 - 107299_5, 2020/06
Times Cited Count:6 Percentile:60.71(Nuclear Science & Technology)A large amount of general steel waste is generated during decommissioning and dismantling of nuclear facilities. Very low-contaminated radioactive waste, whose radioactivity is below clearance level, generated from the demolition process may be reused for general use. We examined the feasibility of the clearance verification system for uranium waste. The relative error of uranium determination was within 30% for 1 g of uranium when measuring steel materials (angle bar, channel steel, pipe steel, square steel tube, fragments of metal tube).
Ohashi, Yusuke; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 321(2), p.683 - 691, 2019/08
Times Cited Count:1 Percentile:11.15(Chemistry, Analytical)Sediment waste generated from the waste solution of uranium handling facilities contains uranium and iron. In this study, we examined methods to selectively separate uranium from the waste without using nitric acid. Uranium dissolved in 0.5 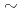 2.5M sulfuric acid, 1M hydrochloric acid, and 5M perchloric acid was selectively precipitated by adding oxalic acid. In addition, when oxalic acid and NCP are added together in 2.5M sulfuric acid, it seems that new insoluble uranium compounds are formed. Consequently, precipitation ratios of uranium was improved. Uranium was selectively precipitated from the sulfuric acid solution containing Fe and Al, and the precipitation ratio of uranium became 99%.
2.5M sulfuric acid, 1M hydrochloric acid, and 5M perchloric acid was selectively precipitated by adding oxalic acid. In addition, when oxalic acid and NCP are added together in 2.5M sulfuric acid, it seems that new insoluble uranium compounds are formed. Consequently, precipitation ratios of uranium was improved. Uranium was selectively precipitated from the sulfuric acid solution containing Fe and Al, and the precipitation ratio of uranium became 99%.
Yokoyama, Kaoru; Ohashi, Yusuke
Applied Radiation and Isotopes, 145, p.19 - 23, 2019/03
Times Cited Count:5 Percentile:48.99(Chemistry, Inorganic & Nuclear)Dismantled materials generated from nuclear facilities are reused or directed to repository sites. If scrap metals with complicated shapes can be cleared, the amounts of radioactive waste can be reduced. A clearance verification system is constructed to determine the amount of uranium in decontaminated metals in a drum using the 1.001 MeV gamma rays of 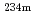 Pa, produced in the decay of
Pa, produced in the decay of  U. The experimental study with simulated waste drums demonstrated that the quantification errors of uranium fall within 25% for 0.5g of uranium.
U. The experimental study with simulated waste drums demonstrated that the quantification errors of uranium fall within 25% for 0.5g of uranium.
Onoe, Hironori; Yamamoto, Shinya*; Kohashi, Akio; Ozaki, Yusuke; Sakurai, Hideyuki*; Masumoto, Kiyoshi*
JAEA-Research 2018-003, 84 Pages, 2018/06
In this study, numerical experiments considered hydrogeological structures, which has high heterogeneity around the Mizunami Underground Research Laboratory and inverse analysis using in-situ data were carried out. The results showed that concentration of hydrogeological structure to be estimated and location of monitoring point is important for application of inverse analysis. Furthermore, it is concluded that inverse analysis using hydraulic response due to pumping test is effective for hydrogeological characterization.
Ohashi, Yusuke; Tanaka, Yoshio; Tsunashima, Yasumichi; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 54(3), p.382 - 390, 2017/03
Times Cited Count:9 Percentile:61.27(Nuclear Science & Technology)Sludge-like uranium wastes (SUWs) have been generated with neutralization of acidic aqueous solutions used for decontamination of metal wastes containing a large amount of iron. We have examined the method for recovering uranium from such SUWs using 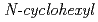 -2-pyrrolidone (NCP) as a precipitate. As a result, it was found that precipitation ratios (PRs) of uranium in the solutions prepared by dissolving SUWs in HNO
-2-pyrrolidone (NCP) as a precipitate. As a result, it was found that precipitation ratios (PRs) of uranium in the solutions prepared by dissolving SUWs in HNO is 97.7% at [NCP]/[U(VI)] = 20, and that the PRs of iron, aluminum, fluorine, and sulfate species are less than 1%. This indicates that uranium species are precipitated selectively. The content ratios of U, Fe, Ca, F, and S in the materials after calcining precipitates obtained at [NCP]/[U(VI)] = 20 were in accordance with the conditions of uranium ore concentrate. From these results, it is expected that highly purified uranium can be efficiently recovered from SUWs by using NCP as the precipitant.
is 97.7% at [NCP]/[U(VI)] = 20, and that the PRs of iron, aluminum, fluorine, and sulfate species are less than 1%. This indicates that uranium species are precipitated selectively. The content ratios of U, Fe, Ca, F, and S in the materials after calcining precipitates obtained at [NCP]/[U(VI)] = 20 were in accordance with the conditions of uranium ore concentrate. From these results, it is expected that highly purified uranium can be efficiently recovered from SUWs by using NCP as the precipitant.
Ohashi, Yusuke; Harada, Masayuki*; Asanuma, Noriko*; Ando, Shion; Tanaka, Yoshio; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 311(1), p.491 - 502, 2017/01
Times Cited Count:1 Percentile:10.62(Chemistry, Analytical)In order to assess the feasibility of method for recovering U from wastes containing uranium (scrap uranium) using polyvinylpolypyrrolidone (PVPP) adsorbent, we have examined the adsorption and desorption behavior of metal species in HCl aqueous solutions dissolving scrap uranium. It was found that the U(VI) species are selectively adsorbed onto PVPP regardless of the presence of a large amount of Na(I) and Al(III), that the adsorbed U(VI) species are desorbed from PVPP column selectively by water. Pure uranium was efficiently recovered from the eluates. From these results, the PVPP resin is expected to be used as the adsorbent in the treatment process of scrap uranium.
Ohashi, Yusuke; Asanuma, Noriko*; Harada, Masayuki*; Tanaka, Yoshio; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 309(2), p.627 - 636, 2016/08
Times Cited Count:7 Percentile:55.03(Chemistry, Analytical)As one of methods for recovering uranium from the uranium-bearing wastes, we have proposed the electrolytic deposition method using choline chloride-urea (CCU) which is known as an ambient temperature molten salt. More than 92% of uranium components in inactivated alumina and spent sodium fluoride adsorbent was dissolved into CCU solution. Cyclic voltammograms (CVs) of the solutions prepared by dissolving uranium-bearing wastes in CCU were measured in the potential range of -2.0 to 1.1 V (vs. Ag/AgCl). The one reduction peak was observed around -0.7 V for all solutions. Based on the results of CVs, bulk electrolyses of the solutions dissolving uranium-bearing wastes were also carried out at -1.5V at 80  C. The deposits were formed on a carbon electrode as cathode. Consequently, we confirmed that CCU is effective media for recovering uranium selectively from uranium-bearing waste.
C. The deposits were formed on a carbon electrode as cathode. Consequently, we confirmed that CCU is effective media for recovering uranium selectively from uranium-bearing waste.
Ohashi, Yusuke; Harada, Masayuki*; Asanuma, Noriko*; Ikeda, Yasuhisa*
Journal of Nuclear Materials, 464, p.119 - 127, 2015/09
Times Cited Count:15 Percentile:77.56(Materials Science, Multidisciplinary)In order to examine feasibility of the electrochemical deposition method for recovering uranium from the solid wastes contaminated with uranium using ionic liquid as electrolyte, we have studied the electrochemical behavior of each solution prepared by soaking the spent NaF adsorbents and the steel waste contaminated with uranium in BMICl (1-butyl-3-methyl- imidazolium chloride). The uranyl(VI) species in BMICl solutions were found to be reduced to U(V) irreversibly around -0.8 to -1.3 V vs. Ag/AgCl. Based on the electrochemical data, we have performed potential controlled electrolysis of each solution at -1.5 V vs. Ag/AgCl. Black deposit was obtained, and their composition analyses suggest that the deposit is the mixtures of U(IV) and U(VI) compounds containing O, F, Cl, and N elements. From the present study, it is expected that the solid wastes contaminated with uranium can be decontaminated by treating them in BMICl and the dissolved uranium species are recovered electrolytically.
Ohashi, Yusuke; Nomura, Mitsuo; Tsunashima, Yasumichi; Ando, Shion; Sugitsue, Noritake; Ikeda, Yasuhisa*; Tanaka, Yoshio
Journal of Nuclear Science and Technology, 51(2), p.251 - 265, 2014/02
Times Cited Count:9 Percentile:53.31(Nuclear Science & Technology)Sludge-like uranium-bearing wastes generated from uranium refining and conversion R&D facilities are stored at the Ningyo-toge Environmental Engineering Center. We have proposed an aqueous process for recovering uranium from spent filter aid and CaF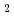 precipitate using hydrochloric acid. The distributions of the dissolved species in the sample solutions at different pH levels were calculated using the chemical equilibrium modeling system. Calculated results of fluorine contents of recovered uranium were compared with the experimental results. The fluorine content in the recovered uranium decreased as the aluminum concentration of the solution increased. On the other hand, uranium of spent filter aid was recovered selectively. The size of the particles of recovered uranium tends to decrease with increasing pH in the precipitation treatments. Also, the uranium concentration of the precipitate generated by the neutralization of the barren solution falls below 1 Bq/g.
precipitate using hydrochloric acid. The distributions of the dissolved species in the sample solutions at different pH levels were calculated using the chemical equilibrium modeling system. Calculated results of fluorine contents of recovered uranium were compared with the experimental results. The fluorine content in the recovered uranium decreased as the aluminum concentration of the solution increased. On the other hand, uranium of spent filter aid was recovered selectively. The size of the particles of recovered uranium tends to decrease with increasing pH in the precipitation treatments. Also, the uranium concentration of the precipitate generated by the neutralization of the barren solution falls below 1 Bq/g.
Nagano, Tetsushi; Yanase, Nobuyuki; Naganawa, Hirochika; Mitamura, Hisayoshi; Hanzawa, Yukiko; Mita, Yutaka; Kanda, Nobuhiro; Ohashi, Yusuke; Endo, Yuji; Matsubara, Tatsuo
Nihon Genshiryoku Gakkai Wabun Rombunshi, 12(4), p.277 - 285, 2013/12
no abstracts in English
Ohashi, Yusuke; Tsunashima, Yasumichi; Tanaka, Yoshio; Sugitsue, Noritake
Proceedings of 5th International Conference and Exhibition on Decommissioning Challenges; Industrial Reality and Prospects (CD-ROM), 10 Pages, 2013/04
Technologies for uranium refining and conversion for production of UF had been developed in Ningyo-Toge environmental engineering center. As a result, a significant sludge like uranium bearing waste and adsorbent was generated. These wastes total 1500 tons. They are dissolved using hydrochloric acid and dissolved uranium is recovered as uranium peroxide. Impurities in uranium peroxide and uranium content were compared with the requirement defined by ASTM. Consequently, highly pure uranium which met the requirement was recovered at low pH. The uranium remaining in the solution was removed using chelating resin in order to decrease uranium radioactivity of the neutralized precipitate that is generated later in the process. It is confirmed that aluminum in the neutralized precipitate is recovered selectively using sodium hydroxide.
had been developed in Ningyo-Toge environmental engineering center. As a result, a significant sludge like uranium bearing waste and adsorbent was generated. These wastes total 1500 tons. They are dissolved using hydrochloric acid and dissolved uranium is recovered as uranium peroxide. Impurities in uranium peroxide and uranium content were compared with the requirement defined by ASTM. Consequently, highly pure uranium which met the requirement was recovered at low pH. The uranium remaining in the solution was removed using chelating resin in order to decrease uranium radioactivity of the neutralized precipitate that is generated later in the process. It is confirmed that aluminum in the neutralized precipitate is recovered selectively using sodium hydroxide.
Hata, Haruhi; Yokoyama, Kaoru; Tsunashima, Yasumichi; Ohashi, Yusuke; Koga, Osamu; Sugitsue, Noritake
JAEA-Research 2011-022, 35 Pages, 2011/09
For disposal of Very low-level radioactive Waste (VLLW) from nuclear related facilities, one of important factors for safety assessment is the characteristics of elution. As for VLLW from the nuclear power plant, concrete pit and trench disposals have been performed and the evaluation methods for the characteristics have been established. On the other hand, as for the uranium waste, the concept on how to test the elution characteristics is not shown yet. Based on these circumstances, preliminary tests have been conducted to study elution characteristics of uranium waste. The results show that the important factors for the uranium elution are how uranium exists in waste. In addition, the elution characteristics also depend on the precipitation amount on the disposal site. Therefore, to assess the elution rate from uranium waste, these factors must be considered.

Ohashi, Yusuke; Asanuma, Noriko*; Harada, Masayuki*; Wada, Yukio*; Matsubara, Tatsuo; Ikeda, Yasuhisa*
Journal of Nuclear Science and Technology, 46(8), p.771 - 775, 2009/08
Most of the metal or bed material wastes generated from uranium enrichment facilities or uranium refining and conversion plants are contaminated by uranium fluoride compounds such as UF . The UF
. The UF powder was completely dissolved in BMICl(1-buthyl-3-methylimidazolium chloride). The uranium concentrations of metal waste dropped below the temporary proposed clearance level (1.0 Bq/g) using BMICl. In the cyclic voltammogram of BMICl solution when dissolving UF
powder was completely dissolved in BMICl(1-buthyl-3-methylimidazolium chloride). The uranium concentrations of metal waste dropped below the temporary proposed clearance level (1.0 Bq/g) using BMICl. In the cyclic voltammogram of BMICl solution when dissolving UF , uncoupled reduction and oxidation peaks were observed and the reduction peak was considered to correspond to the reduction of uranyl(VI) + e
, uncoupled reduction and oxidation peaks were observed and the reduction peak was considered to correspond to the reduction of uranyl(VI) + e
 uranyl(V) followed by further reduction to UO
uranyl(V) followed by further reduction to UO .
.
Ohashi, Yusuke; Tsunashima, Yasumichi; Murata, Masato
Proceedings of 2nd International Conference on Sustainable Development through Nuclear Research and Education (Nuclear 2009) (CD-ROM), p.161 - 168, 2009/05
Solid and liquid radioactive waste derived from various research such as technology development for uranium refining and conversion stored currently totals 1500tons. NaF waste, used as an absorbent of UF contains about 20-30wt% uranium. CaF
contains about 20-30wt% uranium. CaF waste, which is generated from the disposal of waste water including fluoride, contains up to 20wt% uranium. Most of these are classified as intermediate disposal waste. Hence it is necessary to recover uranium to dispose waste as a shallow ground disposal waste. Dissolution experiments were carried out by charging CaF
waste, which is generated from the disposal of waste water including fluoride, contains up to 20wt% uranium. Most of these are classified as intermediate disposal waste. Hence it is necessary to recover uranium to dispose waste as a shallow ground disposal waste. Dissolution experiments were carried out by charging CaF waste and NaF waste into 1N HCl. The dissolution rates of CaF
waste and NaF waste into 1N HCl. The dissolution rates of CaF waste and NaF waste were 99.8% and 100% respectively. The recovery rates of uranium from CaF
waste and NaF waste were 99.8% and 100% respectively. The recovery rates of uranium from CaF solution and NaF solution were 98.2% and 99.7% respectively. It was confirmed that we could dispose these waste reasonably.
solution and NaF solution were 98.2% and 99.7% respectively. It was confirmed that we could dispose these waste reasonably.
Ohashi, Yusuke
no journal, ,
We propose ways to use ionic liquid as reaction media in order to separate uranium and to recover uranium from contaminated materials that is generated from nuclear fuel institutions. By using BMICl and eutectic mixtures of choline chloride-urea we did decontamination tests of waste NaF adsorption materials and metal wastes. We confirmed the solubility of uranium included in NaF into ionic liquids. The decontamination effect of ionic liquids to metal wastes were also confirmed. As a result it was confirmed that 86% of uranium included in NaF is soluble in BMICl after 3 hours soaking. 64% of uranium included in NaF was soluble in eutectic mixtures of choline chloride-urea under the same condition. On the other hand, after we have soaked contaminated metal materials into BMICl for 3 hours, the density of surface radioactivity became lower than 1/10 of levels at which we can bring it out from restricted areas.
Ohashi, Yusuke; Asanuma, Noriko*; Harada, Masayuki*; Ikeda, Yasuhisa*
no journal, ,
In the cyclic voltammogram of BMICl solution when dissolving metal waste contaminated with UF , uncoupled reduction peak and a pair of oxidation-reduction peaks were observed around -1.0V and 0V. The reduction peak was considered to correspond to the reduction of U(VI)
, uncoupled reduction peak and a pair of oxidation-reduction peaks were observed around -1.0V and 0V. The reduction peak was considered to correspond to the reduction of U(VI)  U(V). A pair of peaks were considered to be the reversible peak of Fe(III)
U(V). A pair of peaks were considered to be the reversible peak of Fe(III)  Fe(II). After the electrolysis of the solution, the working electrode was washed by ethanol. From the results of XPS measurement, uranium component was observed on the surface of the electrode though no Fe conponent was observed. Hence we would expect that the uranium component could be recovered by electrolysis from the solution generated in the decontamination treatment of the steel wastes contaminated with UF
Fe(II). After the electrolysis of the solution, the working electrode was washed by ethanol. From the results of XPS measurement, uranium component was observed on the surface of the electrode though no Fe conponent was observed. Hence we would expect that the uranium component could be recovered by electrolysis from the solution generated in the decontamination treatment of the steel wastes contaminated with UF in BMICl.
in BMICl.