Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nanjo, Kotaro; Shiotsu, Hiroyuki; Maruyama, Yu; Sugiyama, Tomoyuki; Okamoto, Koji*
Journal of Nuclear Science and Technology, 60(7), p.816 - 823, 2023/07
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Nanjo, Kotaro; Ishikawa, Jun; Sugiyama, Tomoyuki; Pellegrini, M.*; Okamoto, Koji*
Journal of Nuclear Science and Technology, 59(11), p.1407 - 1416, 2022/11
Times Cited Count:7 Percentile:73.20(Nuclear Science & Technology)Okumura, Keisuke; Sugino, Kazuteru; Kojima, Kensuke; Jin, Tomoyuki*; Okamoto, Tsutomu; Katakura, Junichi*
JAEA-Data/Code 2012-032, 148 Pages, 2013/03
A set of cross section libraries for the isotope generation and depletion calculation code ORIGEN2 was produced by using recent nuclear data JENDL-4.0. In this new library (ORLIBJ40), neutron-induced cross sections, fission product yields, isomeric ratios and half-lives were updated. ORLIBJ40 includes 24 libraries for typical UO or MOX fuels of PWR and BWR. In addition, it includes 36 libraries for various fast reactor fuels. ORLIBJ40 was applied to the post irradiation examination analyses of LWR nuclear spent fuels. As a result, it was confirmed that improvements were achieved especially for inventory and radioactivity estimations of minor actinides (Am and Cm isotopes) and fission products sensitive to cross sections (Eu and Sm isotopes) and for long-lived fission products (
or MOX fuels of PWR and BWR. In addition, it includes 36 libraries for various fast reactor fuels. ORLIBJ40 was applied to the post irradiation examination analyses of LWR nuclear spent fuels. As a result, it was confirmed that improvements were achieved especially for inventory and radioactivity estimations of minor actinides (Am and Cm isotopes) and fission products sensitive to cross sections (Eu and Sm isotopes) and for long-lived fission products (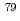 Se, etc.), compared with other existing ORIGEN2 libraries.
Se, etc.), compared with other existing ORIGEN2 libraries.
Kobayashi, Toru*; Harata, Yasuo*; Matsufuji, Naruhiro*; Hasegawa, Tomoyuki*; Endo, Akira; Moribayashi, Kengo; Akahane, Keiichi*; Uehara, Shuzo*; Imahori, Yoshio*; Kato, Yo*; et al.
JAEA-Review 2006-002, 101 Pages, 2006/02
This report provides an analysis of the results of the survey conducted among field experts regarding the data on atoms, molecules, and atomic nuclei used in medical applications. The important results are summarized as follows: First, the importance of the basic data for disciplines involved in medical research, i.e. physics and engineering, chemistry, pharmacology, biology, and the related data which are applied directly in medicine were identified. The related data are of greater importance in direct medical application compared to conventional basic data. Therefore, the data related to biology should be prepared in consideration of their convenient usage. Second, regarding the fundamental data on atoms, molecules and atomic nuclei related to medicine, the present data was able to approximately cope with the demands of many medical cases that needed data on quality, quantity, precision, etc. However, we found situations particularly in the IT community where comprehensively organized data was urgently needed. The data to be used for practical implementation must contain the specialized data for medical physics and biology. Finally, the significance of the continuity in the planned completion of the basic data was confirmed for the development of the associated fields. The expansion and completion of basic data should be done continuously and effectively while considering the limitation in resources and manpower.
Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Proceedings of the National Academy of Sciences of the United States of America, 103(9), p.3135 - 3140, 2006/02
Times Cited Count:99 Percentile:84.85(Multidisciplinary Sciences)A crystal structure of the signaling complex between human granulocyte colony-stimulating factor (GCSF), and a ligand binding region of GCSF receptor (GCSF-R), has been determined to 2.8 resolution. The GCSF:GCSF-R complex formed a 2:2 stoichiometry via a cross-over interaction between the Ig-like domains of GCSF-R and GCSF. The conformation of the complex is quite different to that between human GCSF and the CRH domain of mouse GCSF-R, but similar to that of the interleukin-6 (IL-6)/gp130 signaling complex. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
resolution. The GCSF:GCSF-R complex formed a 2:2 stoichiometry via a cross-over interaction between the Ig-like domains of GCSF-R and GCSF. The conformation of the complex is quite different to that between human GCSF and the CRH domain of mouse GCSF-R, but similar to that of the interleukin-6 (IL-6)/gp130 signaling complex. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Honjo, Eijiro; Tamada, Taro; Maeda, Yoshitake*; Koshiba, Takumi*; Matsukura, Yasuko*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
Acta Crystallographica Section F, 61(8), p.788 - 790, 2005/08
Times Cited Count:7 Percentile:54.61(Biochemical Research Methods)Granulocyte colony-stimulating factor (GCSF) receptor receives signals for regulating the proliferation and differentiation of the precursor cells of granulocytes. The complex composed of two GCSFs and two GCSF receptors was crystallized. The crystal of the complex was grown in 1.0 M sodium formate and 0.1 M sodium acetate (pH4.6). It belongs to the space group  4
4 2
2 2 (or its enantiomorph
2 (or its enantiomorph  4
4 2
2 2) with unit cell dimensions of
2) with unit cell dimensions of  =
=  = 110.1
= 110.1  ,
,  = 331.8
= 331.8  . However, the diffraction data from the crystal beyond 5
. However, the diffraction data from the crystal beyond 5  resolution could not be collected. Since the heterogeneity of GCSF receptor seems to interrupt growth of good quality crystals, the GCSF receptor was fractionated by achromatography. Crystals of GCSF/fractionated GCSF receptor complex were grown as a new crystal form in 0.2 M ammonium phosphate. The new crystal diffracts beyond 3.0
resolution could not be collected. Since the heterogeneity of GCSF receptor seems to interrupt growth of good quality crystals, the GCSF receptor was fractionated by achromatography. Crystals of GCSF/fractionated GCSF receptor complex were grown as a new crystal form in 0.2 M ammonium phosphate. The new crystal diffracts beyond 3.0  resolution and belongs to space group
resolution and belongs to space group  3
3 21 (or its enantiomorph
21 (or its enantiomorph  3
3 21) with unit-cell parameters
21) with unit-cell parameters  =
=  = 134.8,
= 134.8,  = 105.7
= 105.7  .
.
Tsuji, Nobumasa*; Okamoto, Futoshi*; Murakami, Tomoyuki; Kunitomi, Kazuhiko
no journal, ,
no abstracts in English
Kobayashi, Toru*; Harata, Yasuo*; Matsufuji, Naruhiro*; Hasegawa, Tomoyuki*; Endo, Akira; Moribayashi, Kengo; Akahane, Keiichi*; Uehara, Shuzo*; Imahori, Yoshio*; Kato, Yo*; et al.
no journal, ,
no abstracts in English
Tamada, Taro; Honjo, Eijiro; Maeda, Yoshitake*; Okamoto, Tomoyuki*; Ishibashi, Matsujiro*; Tokunaga, Masao*; Kuroki, Ryota
no journal, ,
We have succeeded in crystallization of 2:2 complex between human granulocyte colony-stimulating factor (hGCSF) and the Ig-like and CRH domains of human GCSF-R (hGCSF-R) and determined its tertiary structure by X-ray crystallography at 2.8  resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6
resolution. The signaling 2:2 complex is formed via cross-over interactions between the Ig-like domain of hGCSF-R and the neighboring hGCSF, forming a two-fold axis of crystallographic symmetry. This conformation is quite different from that of the heterogeneous mGCSF-R complex, and more closely resembles the 2:2:2 active assembly of human interleukin-6 (IL-6), human IL-6  -receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
-receptor and human gp130 (which is a shared signal transducing receptor for several cytokines), and the 2:2 assembly of viral IL-6 and human gp130. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.