Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Aoyagi, Noboru; Motokawa, Ryuhei; Okumura, Masahiko; Ueda, Yuki; Saito, Takumi*; Nishitsuji, Shotaro*; Taguchi, Tomitsugu*; Yomogida, Takumi; Sazaki, Gen*; Ikeda, Atsushi
Communications Chemistry (Internet), 7, p.128_1 - 128_13, 2024/06
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)no abstracts in English
Kobayashi, Keita; Okumura, Masahiko; Nakamura, Hiroki; Itakura, Mitsuhiro; Machida, Masahiko; Urata, Shingo*; Suzuya, Kentaro
Scientific Reports (Internet), 13, p.18721_1 - 18721_12, 2023/11
Times Cited Count:1 Percentile:40.78(Multidisciplinary Sciences)The first sharp peak diffraction peak (FSDP) in the structure factor of amorphous materials is thought to reflect the medium-range order structure in amorphous materials, and the structural origin of the FSDP has been a subject of ongoing debate. In this study, we employed machine learning molecular dynamics (MLMD) with nearly first-principles calculation accuracy to investigate the structural origin of the FSDP in high-density silica glass. First, we successfully reproduced various experimental data of high-density silica glass using MLMD. Furthermore, we revealed that the development (or reduction) of the FSDP in high-density silica glass is characterized by the deformation behavior of ring structures in Si-O covalent bond networks under compression.
Yamaguchi, Akiko; Okumura, Masahiko; Takahashi, Yoshio*
Isotope News, (789), p.20 - 23, 2023/10
Radium is a radioactive element produced from uranium and thorium and is important for environmental contamination issues around uranium mines and for geological disposal. In addition, radium is used in radiometric dating and cancer therapy, making it important not only in environmental chemistry but also in many other fields, including geochemistry and nuclear medicine. However, because radium is a radioactive element with no stable isotopes, spectroscopic measurement of radium is difficult, and little information at the molecular level has been obtained so far. In this study, we have clarified the molecular-level information of hydrated radium for the first time in the world by combining extended X-ray absorption fine structure (EXAFS) measurements and first-principles molecular dynamics simulations.

 values calculated from radiocesium interception potential
values calculated from radiocesium interception potentialUno, Koichiro*; Nakao, Atsushi*; Okumura, Masahiko; Yamaguchi, Akiko; Kogure, Toshihiro*; Yanai, Junta*
Nihon Dojo Hiryo Gaku Zasshi, 94(5), p.376 - 384, 2023/10
Radiocesium interception potential (RIP) has been widely used as a quantitative indicator of cesium (Cs) adsorption capacity of soil, but it has been found that RIP does not always correlate with the distribution coefficient (
 ) of Cs in the actual environment. In order to clarify the cause of this discrepancy, we measured Kd using more realistic solutions, compared it with RIP, and evaluated the mineral structure. As a result, it was found that the concentration of competing cations, such as potassium and ammonium ions, and the structural change of the mineral itself are important.
) of Cs in the actual environment. In order to clarify the cause of this discrepancy, we measured Kd using more realistic solutions, compared it with RIP, and evaluated the mineral structure. As a result, it was found that the concentration of competing cations, such as potassium and ammonium ions, and the structural change of the mineral itself are important.
Machida, Masahiko; Yamada, Susumu; Kim, M.; Okumura, Masahiko; Miyamura, Hiroko; Shikaze, Yoshiaki; Sato, Tomoki*; Numata, Yoshiaki*; Tobita, Yasuhiro*; Yamaguchi, Takashi; et al.
RIST News, (69), p.2 - 18, 2023/09
The contamination of radioactive materials leaked from the reactor has resulted in numerous hot spots in the Fukushima Daiichi Nuclear Power Station (1F) building, posing obstacles to its decommissioning. In order to solve this problem, JAEA has conducted research and development of the digital technique for inverse estimation of radiation source distribution and countermeasures against the estimated source in virtual space for two years from 2021 based on the subsidy program "Project of Decommissioning and Contaminated Water Management" performed by the funds from the Ministry of Economy, Trade and Industry. In this article, we introduce the results of the project and the plan of the renewal project started in April 2023. For the former project, we report the derivative method for LASSO method considering the complex structure inside the building and the character of the source and show the result of the inverse estimation using the method in the real reactor building. Moreover, we explain the platform software "3D-ADRES-Indoor" which integrates these achievements. Finally, we introduce the plan of the latter project.
Mori, Hideki*; Tsuru, Tomohito; Okumura, Masahiko; Matsunaka, Daisuke*; Shiihara, Yoshinori*; Itakura, Mitsuhiro
Physical Review Materials (Internet), 7(6), p.063605_1 - 063605_8, 2023/06
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)The introduction of obstacles (e.g., precipitates) for controlling dislocation motion in molecular structures is a prevalent method for designing the mechanical strength of metals. Owing to the nanoscale size of the dislocation core ( 1 nm), atomic modeling is required to investigate the interactions between the dislocation and obstacles. However, conventional empirical potentials are not adequately accurate, in contrast to the calculations based on density functional theory (DFT). Therefore, the atomic-level details of the interactions between the dislocations and obstacles remain unclarified. To this end, this study applied an artificial neural network (ANN) framework to construct an atomic potential by leveraging the high accuracy of DFT. Using the constructed ANN potential, we investigated the dynamic interaction between the
1 nm), atomic modeling is required to investigate the interactions between the dislocation and obstacles. However, conventional empirical potentials are not adequately accurate, in contrast to the calculations based on density functional theory (DFT). Therefore, the atomic-level details of the interactions between the dislocations and obstacles remain unclarified. To this end, this study applied an artificial neural network (ANN) framework to construct an atomic potential by leveraging the high accuracy of DFT. Using the constructed ANN potential, we investigated the dynamic interaction between the 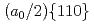 edge dislocation and obstacles in BCC iron. When the dislocation crossed the void, an ultrasmooth and symmetric half-loop was observed for the bowing-out dislocation. Except for the screw dislocation, the Peierls stress of all the dislocations predicted using the ANN was less than 100 MPa. More importantly, the results confirmed the formation of an Orowan loop in the interaction between a rigid sphere and dislocation. Furthermore, we discovered a phenomenon in which the Orowan loop disintegrated into two small loops during its interaction with the rigid sphere and dislocation.
edge dislocation and obstacles in BCC iron. When the dislocation crossed the void, an ultrasmooth and symmetric half-loop was observed for the bowing-out dislocation. Except for the screw dislocation, the Peierls stress of all the dislocations predicted using the ANN was less than 100 MPa. More importantly, the results confirmed the formation of an Orowan loop in the interaction between a rigid sphere and dislocation. Furthermore, we discovered a phenomenon in which the Orowan loop disintegrated into two small loops during its interaction with the rigid sphere and dislocation.
Kobayashi, Keita; Nakamura, Hiroki; Itakura, Mitsuhiro; Machida, Masahiko; Okumura, Masahiko
Materia, 62(3), p.175 - 181, 2023/03
no abstracts in English
Kobayashi, Keita; Yamaguchi, Akiko; Okumura, Masahiko
Applied Clay Science, 228, p.106596_1 - 106596_11, 2022/10
Times Cited Count:6 Percentile:78.82(Chemistry, Physical)no abstracts in English
 molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:9 Percentile:68.02(Multidisciplinary Sciences)no abstracts in English
Kobayashi, Keita; Okumura, Masahiko; Nakamura, Hiroki; Itakura, Mitsuhiro; Machida, Masahiko; Cooper, M. W. D.*
Scientific Reports (Internet), 12(1), p.9808_1 - 9808_11, 2022/06
Times Cited Count:8 Percentile:71.45(Multidisciplinary Sciences)no abstracts in English
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
Okita, Taira*; Terayama, Satoshi*; Tsugawa, Kiyoto*; Kobayashi, Keita; Okumura, Masahiko; Itakura, Mitsuhiro; Suzuki, Katsuyuki*
Computational Materials Science, 202, p.110865_1 - 110865_9, 2022/02
Times Cited Count:7 Percentile:45.98(Materials Science, Multidisciplinary)Okumura, Masahiko
Chikyu Kagaku, 55(4), p.110 - 121, 2021/12
no abstracts in English
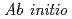 molecular dynamics simulations using the SCAN meta-GGA density functional and EXAFS spectroscopy studies
molecular dynamics simulations using the SCAN meta-GGA density functional and EXAFS spectroscopy studiesYamaguchi, Akiko; Kobayashi, Keita; Takahashi, Yoshio*; Machida, Masahiko; Okumura, Masahiko
Chemical Physics Letters, 780, p.138945_1 - 138945_5, 2021/10
Times Cited Count:6 Percentile:49.65(Chemistry, Physical)no abstracts in English
Kobayashi, Keita; Nakamura, Hiroki; Yamaguchi, Akiko; Itakura, Mitsuhiro; Machida, Masahiko; Okumura, Masahiko
Computational Materials Science, 188, p.110173_1 - 110173_14, 2021/02
Times Cited Count:15 Percentile:73.05(Materials Science, Multidisciplinary)no abstracts in English
Nagao, Fumiya; Niizato, Tadafumi; Sasaki, Yoshito; Ito, Satomi; Watanabe, Takayoshi; Dohi, Terumi; Nakanishi, Takahiro; Sakuma, Kazuyuki; Hagiwara, Hiroki; Funaki, Hironori; et al.
JAEA-Research 2020-007, 249 Pages, 2020/10
The accident of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. occurred due to the Great East Japan Earthquake, Sanriku offshore earthquake, of 9.0 magnitude and the accompanying tsunami. As a result, large amount of radioactive materials was released into the environment. Under these circumstances, Japan Atomic Energy Agency (JAEA) has been conducting "Long-term Assessment of Transport of Radioactive Contaminants in the Environment of Fukushima" concerning radioactive materials released in environment, especially migration behavior of radioactive cesium since November 2012. This report is a summary of the research results that have been obtained in environmental dynamics research conducted by JAEA in Fukushima Prefecture.
Yamaguchi, Akiko; Asano, Ikumi*; Kitagawa, Yuri*; Meng, C.*; Nakao, Atsushi*; Okumura, Masahiko
Proceedings of Joint International Conference on Supercomputing in Nuclear Applications + Monte Carlo 2020 (SNA + MC 2020), p.127 - 130, 2020/10
no abstracts in English
Nagai, Yuki; Okumura, Masahiko; Kobayashi, Keita*; Shiga, Motoyuki
Physical Review B, 102(4), p.041124_1 - 041124_6, 2020/07
Times Cited Count:13 Percentile:64.73(Materials Science, Multidisciplinary)no abstracts in English
Nagai, Yuki; Okumura, Masahiko; Tanaka, Akinori*
Physical Review B, 101(11), p.115111_1 - 115111_12, 2020/03
Times Cited Count:17 Percentile:73.39(Materials Science, Multidisciplinary)no abstracts in English