Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Aoyama, Takahito; Ueno, Fumiyoshi; Sato, Tomonori; Kato, Chiaki; Sano, Naruto; Yamashita, Naoki; Otani, Kyohei; Igarashi, Takahiro
Annals of Nuclear Energy, 214, p.111229_1 - 111229_6, 2025/05
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)Igarashi, Takahiro; Sugawara, Yu*; Otani, Kyohei; Aoyama, Takahito
Tetsu To Hagane, 110(15), p.1244 - 1250, 2024/11
Times Cited Count:0 Percentile:0.00(Metallurgy & Metallurgical Engineering)Using two types of image processing techniques without machine learning, edge extraction processing and keypoint extraction processing, progressively corroded regions under rust layer from images of corroded steel surfaces was extracted. We found that there is a relatively good correlation between the keypoint strength obtained from the keypoint extraction processing for HSL transformed and histogram flattened corroded surface photographs and the corrosion depth after removing rust removal.
Otani, Kyohei; Kato, Chiaki; Igarashi, Takahiro
Corrosion, 79(11), p.1277 - 1286, 2023/11
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.
Igarashi, Takahiro; Otani, Kyohei; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Bosei Kanri, 66(4), p.141 - 145, 2022/04
Metal corrosion is a material deterioration phenomenon based on electrochemical reactions on an atomic scale. In this paper, various methods for acquiring physical properties on metal surfaces using first-principles calculations were described. As examples of applying first-principles calculation to metal corrosion, the effect of hydrogen adsorption on the metal surface on the potential change and the effect of cation atoms in the aqueous solution on the corrosion resistance of the metal were reported.
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(4), p.121 - 125, 2022/04
The effect of solution layer thickness on the atmospheric corrosion of carbon steel was investigated using novel devices fabricated by a 3D printer. These novel devices allowed us to control the solution layer thickness precisely. Potentiodynamic polarization measurements were performed under thickness-controlled solution layer, and oxygen diffusion limiting current density ( ) and anodic current density (
) and anodic current density ( ) were measured. As the solution layer become thinner,
) were measured. As the solution layer become thinner,  increased and
increased and  decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20
decreased. This result indicates that corrosion accelerates when the solution layer becomes thinner. The diffusion coefficient of oxygen was calculated as 3.20 10
10 cm
cm s
s from the relationship between
from the relationship between  and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
and solution layer thickness, and the critical diffusion thickness was estimated to be 0.87 mm.
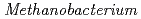 strain induces severe corrosion by retrieving electrons from Fe
strain induces severe corrosion by retrieving electrons from Fe under a freshwater environment
under a freshwater environmentHirano, Shinichi*; Ihara, Sota*; Wakai, Satoshi*; Dotsuta, Yuma; Otani, Kyohei; Kitagaki, Toru; Ueno, Fumiyoshi; Okamoto, Akihiro*
Microorganisms (Internet), 10(2), p.270_1 - 270_12, 2022/02
Times Cited Count:15 Percentile:80.25(Microbiology)To understand the role of methanogens in corrosion under anoxic conditions in freshwater, we investigated the corrosion activities of methanogens in samples collected from groundwater and rivers. We enriched microorganisms that can grow with CO /NaHCO
/NaHCO and Fe
and Fe as the sole carbon source and electron donor, respectively, in ground fresh water. Electrochemical analysis revealed that
as the sole carbon source and electron donor, respectively, in ground fresh water. Electrochemical analysis revealed that 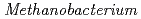 strain can uptake electrons from the cathode at lower than -0.61 V vs SHE and has a redox-active component with electrochemical potential different from those of other previously reported methanogens with extracellular electron transfer ability. This study indicated the corrosion risk by methanogens capable of taking up electrons from Fe
strain can uptake electrons from the cathode at lower than -0.61 V vs SHE and has a redox-active component with electrochemical potential different from those of other previously reported methanogens with extracellular electron transfer ability. This study indicated the corrosion risk by methanogens capable of taking up electrons from Fe in anoxic freshwater environments and the necessity of understanding the corrosion mechanism to contribute to risk diagnosis.
in anoxic freshwater environments and the necessity of understanding the corrosion mechanism to contribute to risk diagnosis.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Otani, Kyohei; Kato, Chiaki
Zairyo To Kankyo, 70(12), p.480 - 486, 2021/12
This is a comprehensive paper of the corrosion of carbon steel in air/solution alternating condition. From cross-sectional observation and analysis of the iron rust layer formed on the surface of carbon steel in the alternating condition, it was found that a multilayered iron rust layer composed of red rust layer ( -FeOOH), rust crust layer (Fe
-FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
Otani, Kyohei; Sakairi, Masatoshi*
Materials Transactions, 62(6), p.815 - 820, 2021/06
Times Cited Count:4 Percentile:21.67(Materials Science, Multidisciplinary)The synergistic effects of metal cations in a solution on the ability of sodium gluconate to inhibit the corrosion of mild steel were investigated quantitatively focus on the parameter Y, which represents the "corrosion inhibitory effect of cations" by immersion and electrochemical tests. The results of the investigations showed that the inhibition ability of gluconates improved with increasing Y value of the metal cations in model freshwater. The electrochemical and surface analyses indicated that gluconate ligands and large-Y metal cations formed a protective film with few defects on the mild steel.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi
Materials Transactions, 62(6), p.763 - 769, 2021/06
Times Cited Count:1 Percentile:4.80(Materials Science, Multidisciplinary)In this study, the iron rust layer formed on the low alloy steel under air-solution alternating conditions was investigated by cross-sectional observation and analysis, and the mechanism of the accelerated corrosion of the steel under alternating conditions was clarified. The observations and analysis showed that the multilayered iron rust layer composed of the red rust layer (FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on the low alloy steel. It can be considered that the multilayered iron rust layer accelerated the cathodic reaction rate of dissolved oxygen under alternating conditions. This acceleration is the reason why the corrosion rate of the low alloy steel under alternating conditions was accelerated.
), and inner rust layer was formed on the low alloy steel. It can be considered that the multilayered iron rust layer accelerated the cathodic reaction rate of dissolved oxygen under alternating conditions. This acceleration is the reason why the corrosion rate of the low alloy steel under alternating conditions was accelerated.
Igarashi, Takahiro; Otani, Kyohei; Kato, Chiaki; Sakairi, Masatoshi*; Togashi, Yusuke*; Baba, Kazuhiko*; Takagi, Shusaku*
ISIJ International, 61(4), p.1085 - 1090, 2021/04
Times Cited Count:2 Percentile:10.12(Metallurgy & Metallurgical Engineering)In order to clarify the effect of metal cations (Zn , Mg
, Mg , Na
, Na ) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn
) in aqueous solution on hydrogen permeation into iron, the amount of hydrogen permeation from iron surface was measured by electrochemical tests with a laser ablation. Moreover, in order to obtain the basic mechanism of hydrogen permeation with metal cation, first-principles calculations were used to acquire the adsorption potential of the metal cation and the electronic state around iron surface. By Zn in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
in solution, anodic reaction on ablated surface by laser irradiation was suppressed. Also, by quantum analysis Zn atoms were chemically bonded stronger than Na and Mg atoms to iron surface. It was suggested that the dissolution reaction of iron was suppressed by the formation of the Zn layer, and that lead suppression of hydrogen permeation into iron.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 69(9), p.246 - 252, 2020/09
The purpose of this study was to investigate the effect of artificial sea water concentration on the corrosion rate of carbon steel under air/solution alternating condition, and to clarify the corrosion mechanism of carbon steel that changes with artificial seawater concentration. Mass measurements showed that the corrosion rate of carbon steel in the alternating condition accelerates with increasing concentration in the concentration region between deionized water to 200 times diluted artificial seawater (ASW), and the corrosion rate decreases with increasing concentration in the concentration region between 20 times diluted ASW to undiluted ASW. It can be considered that the reason why the carbon steel corrosion was suppressed in highly concentrated artificial seawater would Mg ions and Ca ions in the artificial seawater precipitate and cover on the surface due to the increase in pH near the surface by oxygen reduction reaction.
 concentration
concentrationOtani, Kyohei; Islam, M. S.*; Sakairi, Masatoshi*; Kaneko, Akira*
Surface and Interface Analysis, 51(12), p.1207 - 1213, 2019/12
Times Cited Count:1 Percentile:1.58(Chemistry, Physical)The corrosion morphology and composition of corrosion products of A3003 formed in model fresh water with different Zn concentrations were investigated by immersion tests combined with surface observations and analysis using an auger electron spectroscope (AES). The cross-sectional AES observations showed that the thickness of the corrosion product layer formed on A3003 decreases with increases in the Zn
concentrations were investigated by immersion tests combined with surface observations and analysis using an auger electron spectroscope (AES). The cross-sectional AES observations showed that the thickness of the corrosion product layer formed on A3003 decreases with increases in the Zn concentration of the model fresh water. A cross-sectional AES point analysis suggested that the corrosion products formed on the A3003 in the Zn
concentration of the model fresh water. A cross-sectional AES point analysis suggested that the corrosion products formed on the A3003 in the Zn containing model fresh water (Zn
containing model fresh water (Zn
 0.1 mM) have a multi-layer structure, and that the inner of Zn-rich layer would have high corrosion protective properties.
0.1 mM) have a multi-layer structure, and that the inner of Zn-rich layer would have high corrosion protective properties.
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi
Zairyo To Kankyo, 68(8), p.205 - 211, 2019/08
In the present study, the iron rust layer formed on the low ally steel in air-solution alternating condition was investigated by cross-sectional observation and analysis, and the mechanism of accelerated corrosion of the steel in the alternating condition was clarified. Observation and analysis showed that the multi-layered iron rust layer composed of red rust layer (FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on the low alloy steel. It can be considered that the multi-layered iron rust layer accelerated the cathodic reaction rate of the steel in the alternating condition. This acceleration would be the reason why the corrosion rate of the low alloy steel in the alternating condition was accelerated.
), and inner rust layer was formed on the low alloy steel. It can be considered that the multi-layered iron rust layer accelerated the cathodic reaction rate of the steel in the alternating condition. This acceleration would be the reason why the corrosion rate of the low alloy steel in the alternating condition was accelerated.
Otani, Kyohei; Sato, Tomonori; Kaji, Yoshiyuki; Yamamoto, Masahiro
JAEA-Review 2019-007, 15 Pages, 2019/06
Metallic pipes under solid-liquid two phase flow is damaged by collision of solid particle to the pipe walls, and this phenomenon is named "erosion". In the case of the liquid is corrosive solution, further damage is occurred on the pipe walls chemically, and this named "erosion-corrosion". In the Fukushima Daiichi decommissioning project, the fuel debris will be crushed during removal operation of the debris and micro debris particles would be generated. It is estimated that the pipes of the circulating cooling system would be damaged under the solid-liquid two phase flow containing fuel debris particles. For the reason, the previous study about erosion and erosion-corrosion of metallic materials under solid-liquid two phase flow was surveyed. The survey showed that the damage rate by erosion and erosion-corrosion is influence by a lot of parameter in comparison to the corrosion rate which occurred in no-flow solution. Therefore, it is necessary to pay attention to selecting the experimental method and condition before the investigation about erosion-corrosion of metallic materials under solid-liquid two phase flow is carried out.
Islam, M. S.*; Otani, Kyohei; Sakairi, Masatoshi*
ISIJ International, 58(9), p.1616 - 1622, 2018/09
Times Cited Count:7 Percentile:31.22(Metallurgy & Metallurgical Engineering)To elucidate the role of Zn on corrosion of coated steel, the effects of metal cations on the corrosion of carbon steel in the concentrated Cl
on corrosion of coated steel, the effects of metal cations on the corrosion of carbon steel in the concentrated Cl aqueous solutions were studied by immersion tests, surface analysis and electrochemical tests. Among the examined metal cations, Zn
aqueous solutions were studied by immersion tests, surface analysis and electrochemical tests. Among the examined metal cations, Zn showed the significant effect on corrosion inhibition of carbon steel in the Cl
showed the significant effect on corrosion inhibition of carbon steel in the Cl aqueous solution at high concentration. XPS analysis results elucidated that Zn
aqueous solution at high concentration. XPS analysis results elucidated that Zn can remain on the steel surface after immersed in the solutions with Zn
can remain on the steel surface after immersed in the solutions with Zn . EIS measurements showed higher impedance in the solution with Zn
. EIS measurements showed higher impedance in the solution with Zn than other solutions, and the results suggested that Zn
than other solutions, and the results suggested that Zn reduced the defect points in the thin oxide film by forming a metal cation layer. Based on the experimental results, Zn
reduced the defect points in the thin oxide film by forming a metal cation layer. Based on the experimental results, Zn may form a layer on the oxide film that protects the Cl
may form a layer on the oxide film that protects the Cl attack in the solution. The findings demonstrated that the formation of Zn layer on the oxide film is one of the main reason for showing high and longtime corrosion resistance of Zn coated steel substrate.
attack in the solution. The findings demonstrated that the formation of Zn layer on the oxide film is one of the main reason for showing high and longtime corrosion resistance of Zn coated steel substrate.
 aqueous solution
aqueous solutionIslam, M. S.*; Otani, Kyohei; Sakairi, Masatoshi*
Corrosion Science, 140, p.8 - 17, 2018/08
Times Cited Count:17 Percentile:59.10(Materials Science, Multidisciplinary)The corrosion characteristics of SUS304 exposed to 0.5M Cl aqueous solution containing different metal cations were studied with immersion tests, surface analysis and electrochemical tests. The mechanism of corrosion with metal cations was clarified by the XPS analysis results together with the hard and soft acid and base (HSAB) concept and the passive films structure. It is supposed that metal cations with large hardness make a layer by chemical bonding with the passive films. The passive films are protected by the metal cation layer from Cl
aqueous solution containing different metal cations were studied with immersion tests, surface analysis and electrochemical tests. The mechanism of corrosion with metal cations was clarified by the XPS analysis results together with the hard and soft acid and base (HSAB) concept and the passive films structure. It is supposed that metal cations with large hardness make a layer by chemical bonding with the passive films. The passive films are protected by the metal cation layer from Cl attack, and consequently corrosion reactions are inhibited.
attack, and consequently corrosion reactions are inhibited.
大谷 恭平; 加藤 千明
not registered
【課題】本発明は、添加コストや排水の水処理コストが低い防食剤および防食方法を提供することを課題とする。 【解決手段】本発明は、(A)乳酸アルミニウム、および(B)無機酸塩および有機酸塩から選ばれる1種または2種類以上を含有する、水系における金属部材の防食剤、および同防食剤を用いた水系における金属部材の防食方法を提供する。
Otani, Kyohei; Tsukada, Takashi; Ueno, Fumiyoshi; Kato, Chiaki
no journal, ,
It was reported that the corrosion rate of carbon steel was accelerated under the air/solution alternating condition. In this presentation, it is investigated that the effect of oxygen concentration on the corrosion rate of steel under the air/solution alternating condition. As a result, the corrosion rate of carbon steel under the air/solution alternating condition did not increase linearly with increasing oxygen concentration in the air, and the slope in the low concentration range (0-5%) is greater than in the high concentration range (5-20.8%). This suggests that the corrosion rate may be accelerated around the air/solution interface on the inner surface of the PCV if even a small amount of oxygen is introduced into the PCV during the debris removal process.