Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Saito, Junichi; Sagawa, Norihiko; Ohno, Shuji; Hamada, Hirotsugu; Miyahara, Shinya
JNC TN9400 2004-059, 133 Pages, 2004/09
The simplified secondary sodium cooling system, in which utilized lead-bismuth eutectic is utilized as an intermediate coolant has been selected as one of candidate system for the "Feasibility Studies on Commercialized Fast Reactor System (Phase I)". The purpose of this study for the "FS (PhaseII)" is to understand transfer behavior of lead-bismuth in liquid sodium by experiment. The experiments which trickles liquid lead-bismuth into liquid sodium are carried out of under various test temperature and amount of lead-bismuth. The effects of test temperature and amount of lead-bismuth on reaction behavior of sodium and lead-bismuth are clarified from the experimental results. The obtained results from experiments are as follows. (1) The experiment under lower test temperature takes longer time for reaction between sodium and lead-bismuth than that under higher test temperature. It means that test temperature affects the reaction behavior between sodium and lead-bismuth. (2) The amount of dropping lead-bismuth affects an amount and kind of reaction products which are formed by reaction between sodium and lead-bismuth. (3) Reaction heat obtained from the experiments is similar to estimated reaction heat using formation enthalpy of BiNa3 which is one of dominant reaction products.
Saito, Junichi; Takai, Toshihide; Sagawa, Norihiko; Ohno, Shuji; Hamada, Hirotsugu; Miyahara, Shinya
JNC TN9400 2003-057, 87 Pages, 2003/06
The simplified secondary sodium cooling system utilized lead-bismuth eutectic as an intermediate coolant has been selected one of candidate system for the "Feasibility Studies on Commercialized Fast Reactor System (Phase I)". The purpose of this study for the "FS (Phase II)" is to understand transfer behavior of lead-bismuth in liquid sodium by experiment. From the experimental results the fundamental data are obtained for the feasibility evaluation of the simplified secondary sodium cooling system. Twice experiments which trickles liquid lead-bismuth into liquid sodium are carried out at 400 degress centigrade are obtained. (1) From the ICP analyses of L1-1 and L1-2 test, the lead concentration of sodium is higher than the bismuth concentration. This shows that the amount of dissolution of lead into liquid sodium was larger than that of bismuth. This result agrees with data of the previous solubility data in Pb-Na and Bi-Na binary system in sodium. The solid black particles observed in sodium contain a large amount of bismuth. (2) Temperature of liquid sodium rises when the drops of liquid lead-bismuth are added into liquid sodium. The total heating value calculated using temperature rises observed at several parts in equipment is 137 kJ/mol-LBE on L1-2 test. This heat of reaction is promising for leak detection of lead-bismuth into sodium. (3) Many black solid products are observed in sodium taken from L1-1 and L1-2 test apparatus. The reaction products taken from upper location in a sampling finger are very fine and the size was 5 ~ 10 10
10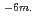 Those from lower location increase in size and the size was 50 ~100
Those from lower location increase in size and the size was 50 ~100 10-6m.
10-6m.
Sagawa, Norihiko*
PNC TJ9613 97-002, 95 Pages, 1997/10
The diffusion coefficient of cesium iodide vapor in rare gases was determined by a modified Stefan's method. The rare gas in a diffusion column was saturated with vapor of the cesium iodide, crystals of which were heated to melt at the bottom of the column. By opening a valve united at a top of the column, the vapor diffusing through the column was transferred with the carrier rare gas to an ionization sensor. The concentration of cesium iodide in the carrier gas was continuously monitored with the sensor. The diffusion coefficient was determined by analyzing the transient response of the concentration. Increasing tendency with temperature is observed in the coefficients obtained in argon, kripton and xenon at temperatures between 631 and 691  C and no significant difference among the coefficients in argon, krypton and xenon.
C and no significant difference among the coefficients in argon, krypton and xenon.
Sagawa, Norihiko*
PNC TJ9613 97-001, 90 Pages, 1997/10
An ionization sensor. which ionizes iodine vapor on a heated filament and collects ionized iodine on a collector at positive potential, was made on an experimental basis. Iodine vapor in rare gas was determined with using the sensor on the line and the characteristic of the sensor was examined. Iodine vapor was generated from iodine crystals in an iodine evaporator and transferred to the sensor with carrier-rare gas. The iodine vapor was continuously monitored by the sensor and trapped in solution of sodium hydroxide. The amount of iodine in the solution was determined by chemical analyses. The integrated value of ion current was compared with the collected amount of the iodine. A proportional relation is observed between the collected amount and the integrated value obtained from the sensor with a platinum collector, but not found between the amount and the value obtained from the sensor with a stainless steel collector.
Miyahara, Shinya; Sagawa, Norihiko; Shimoyama, Kazuhito
Journal of Nuclear Science and Technology, 33(2), p.128 - 133, 1996/00
None
Miyahara, Shinya; Sagawa, Norihiko
Journal of Nuclear Science and Technology, 33(3), p.220 - 222, 1995/00
Times Cited Count:11 Percentile:67.38(Nuclear Science & Technology)None
 C
CMiyahara, Shinya; Sagawa, Norihiko; Sone, Toru; Hara, Hiroshi; Arakawa, Toru*
Journal of Nuclear Science and Technology, 29(4), p.351 - 357, 1992/04
None
Sagawa, Norihiko*
Journal of Nuclear Science and Technology, 28(4), p.305 - 313, 1991/00
None
Miyahara, Shinya; Sone, Toru; Sagawa, Norihiko
Liquid Metal Systems; Material Behavior and Physical Chemistry in Liquid Metal Systems 2, ,
None