Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sghaier, H.*; Sato, Katsuya; Narumi, Issei
Journal of Biomolecular Screening, 16(4), p.457 - 459, 2011/04
Times Cited Count:1 Percentile:5.68(Biochemical Research Methods)
Sghaier, H.*; Sato, Katsuya; Oba, Hirofumi*; Narumi, Issei
African Journal of Biochemistry Research, 4(4), p.111 - 118, 2010/04

Oba, Hirofumi; Sato, Katsuya; Sghaier, H.; Yanagisawa, Tadashi*; Narumi, Issei
Extremophiles, 13(3), p.471 - 479, 2009/05
Times Cited Count:20 Percentile:39.05(Biochemistry & Molecular Biology) possesses a DNA damage response mechanism that acts via the PprI protein to induce RecA and PprA proteins, both of which are necessary in conferring extreme radioresistance. In an effort to further delineate the nature of the DNA damage response mechanism in
possesses a DNA damage response mechanism that acts via the PprI protein to induce RecA and PprA proteins, both of which are necessary in conferring extreme radioresistance. In an effort to further delineate the nature of the DNA damage response mechanism in  , we set out to identify novel components of the PprI-dependent signal transduction pathway in response to radiation stress. Here we demonstrate the discovery of a novel regulatory protein, PprM (a modulator of the PprI-dependent DNA damage response), which is a homolog of cold shock protein (Csp). Disruption of the
, we set out to identify novel components of the PprI-dependent signal transduction pathway in response to radiation stress. Here we demonstrate the discovery of a novel regulatory protein, PprM (a modulator of the PprI-dependent DNA damage response), which is a homolog of cold shock protein (Csp). Disruption of the  gene rendered
gene rendered  significantly sensitive to
significantly sensitive to  -rays. PprM regulates the induction of PprA but not that of RecA. PprM belongs in a distinct clade of a subfamily together with Csp homologs from
-rays. PprM regulates the induction of PprA but not that of RecA. PprM belongs in a distinct clade of a subfamily together with Csp homologs from 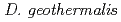 and
and 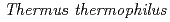 . Purified PprM is present as a homodimer under physiological conditions, as the case with
. Purified PprM is present as a homodimer under physiological conditions, as the case with  CspD. The
CspD. The 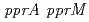 double-disruptant strain exhibited higher sensitivity than the
double-disruptant strain exhibited higher sensitivity than the 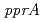 or
or 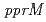 single disruptant strains, suggesting that PprM regulates other hitherto unknown protein(s) important for radioresistance besides PprA. This study strongly suggests that PprM is involved in the radiation response mediated by PprI in
single disruptant strains, suggesting that PprM regulates other hitherto unknown protein(s) important for radioresistance besides PprA. This study strongly suggests that PprM is involved in the radiation response mediated by PprI in 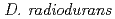 .
.

Oba, Hirofumi; Sato, Katsuya; Kikuchi, Masahiro; Sghaier, H.; Yanagisawa, Tadashi*; Narumi, Issei
JAEA-Review 2008-055, JAEA Takasaki Annual Report 2007, P. 57, 2008/11
no abstracts in English

Sato, Katsuya; Oba, Hirofumi; Sghaier, H.; Narumi, Issei
JAEA-Review 2007-060, JAEA Takasaki Annual Report 2006, P. 92, 2008/03
no abstracts in English
Sghaier, H.; Narumi, Issei; Sato, Katsuya; Oba, Hirofumi; Mitomo, Hiroshi*
Theory in Biosciences, 126(1), p.43 - 45, 2007/03
Times Cited Count:15 Percentile:84.13(Biology)no abstracts in English
 DNA repair-promoting protein; Applications to biotech industry
DNA repair-promoting protein; Applications to biotech industryNarumi, Issei; Oba, Hirofumi; Sghaier, H.; Sato, Katsuya
JAEA-Review 2006-042, JAEA Takasaki Annual Report 2005, P. 69, 2007/02
no abstracts in English

Sato, Katsuya; Oba, Hirofumi; Sghaier, H.; Narumi, Issei
Microbiology, 152(11), p.3217 - 3226, 2006/11
In an effort to gain an insight into the role of LexA2 in the radiation response mechanism, 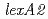 disruptant strain was generated and investigated. The
disruptant strain was generated and investigated. The 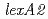 disruptant strains exhibited a much higher resistance to
disruptant strains exhibited a much higher resistance to  rays than the wild-type strain. Furthermore, a luciferase assay showed that
rays than the wild-type strain. Furthermore, a luciferase assay showed that 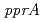 promoter activation was enhanced in the
promoter activation was enhanced in the 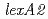 disruptant strain following
disruptant strain following  irradiation. The increase in radioresistance of the
irradiation. The increase in radioresistance of the 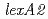 disruptant strain is explained in part by the enhancement of
disruptant strain is explained in part by the enhancement of 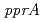 promoter activation.
promoter activation.

Kobayashi, Issei*; Tamura, Takashi*; Sghaier, H.; Narumi, Issei; Yamaguchi, Shotaro*; Umeda, Koichi*; Inagaki, Kenji*
Journal of Bioscience and Bioengineering, 101(4), p.315 - 321, 2006/04
Times Cited Count:37 Percentile:64.70(Biotechnology & Applied Microbiology)Catalase plays a key role in protecting cells against toxic reactive oxygen species. Here we report on the cloning, purification and characterization of a catalase (KatA) from the extremely radioresistant bacterium  . The size of purified
. The size of purified 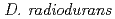 KatA monomer was 65 kDa while gel filtration revealed that the size of the enzyme was 240 kDa, suggesting that KatA formed a homotetramer in solution. Purified KatA displayed a final specific activity of 68,800 U/mg of protein. The catalase activity of KatA was inhibited by sodium azide, sodium cyanide and 3-amino-1, 2, 4-triazole. The absorption spectrum of KatA exhibited a Soret band at 408 nm. The position of the spectral peak remained unchanged following reduction of KatA with dithionite. No peroxidase activity was found for KatA. These results demonstrate that
KatA monomer was 65 kDa while gel filtration revealed that the size of the enzyme was 240 kDa, suggesting that KatA formed a homotetramer in solution. Purified KatA displayed a final specific activity of 68,800 U/mg of protein. The catalase activity of KatA was inhibited by sodium azide, sodium cyanide and 3-amino-1, 2, 4-triazole. The absorption spectrum of KatA exhibited a Soret band at 408 nm. The position of the spectral peak remained unchanged following reduction of KatA with dithionite. No peroxidase activity was found for KatA. These results demonstrate that 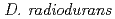 KatA is a typical monofunctional heme-containing catalase. The stability of KatA with respect to H
KatA is a typical monofunctional heme-containing catalase. The stability of KatA with respect to H O
O stress was superior to that of commercially available
stress was superior to that of commercially available 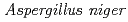 and bovine liver catalases. The relative abundance of KatA in cells in addition to the H
and bovine liver catalases. The relative abundance of KatA in cells in addition to the H O
O resistance property may play a role in the survival strategy of
resistance property may play a role in the survival strategy of 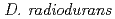 against oxidative damage.
against oxidative damage.
 ; It started out as a radiation resistant organism
; It started out as a radiation resistant organismSghaier, H.; Mitomo, Hiroshi*; Narumi, Issei
Viva Origino, 33(4), p.243 - 257, 2005/12
Previously, analyses of ionizing-radiation-sensitive mutants of  and investigation into the genomic expression profile of the wild-type organism indicated a positive correlation between radiation resistance and desiccation tolerance phenotypes. Mainly because a terrestrial selective force for acquiring resistance to ionizing radiation was unfound, it has been assumed that the radiation resistance of
and investigation into the genomic expression profile of the wild-type organism indicated a positive correlation between radiation resistance and desiccation tolerance phenotypes. Mainly because a terrestrial selective force for acquiring resistance to ionizing radiation was unfound, it has been assumed that the radiation resistance of 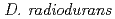 is an incidental phenotype due to its anhydrobiosis defense abilities. Here, following the stratification of the
is an incidental phenotype due to its anhydrobiosis defense abilities. Here, following the stratification of the 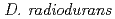 genome, we discuss these issues from evolutionary, genomic, and experimental perspectives. Through the data collected we propose a reconciliatory model wherein radiation resistance is a unique molecular reflection of the early Earth resilience and desiccation tolerance is a mark of cells that colonized land during the Archaean epoch.
genome, we discuss these issues from evolutionary, genomic, and experimental perspectives. Through the data collected we propose a reconciliatory model wherein radiation resistance is a unique molecular reflection of the early Earth resilience and desiccation tolerance is a mark of cells that colonized land during the Archaean epoch.
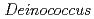 and
and 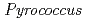 genera
generaSghaier, H.*; Narumi, Issei; Barkallah, I.*
no journal, ,
Narumi, Issei; Oba, Hirofumi*; Sghaier, H.*; Van Long, N.*; Sato, Katsuya*
no journal, ,
no abstracts in English
 retain their small plasmids?; Sequencing, annotation, and analysis
retain their small plasmids?; Sequencing, annotation, and analysisSghaier, H.*; Oba, Hirofumi*; Sato, Katsuya*; Narumi, Issei
no journal, ,
no abstracts in English
Sghaier, H.; Oba, Hirofumi; Sato, Katsuya; Mitomo, Hiroshi*; Narumi, Issei
no journal, ,
The order in which DNA of radiation-resistant prokaryotes has been subjected to constant damage through radiation, elevated temperature, dry, or reactive oxygen species during Earth evolution is important to investigate for a better understanding of environment-genome evolutionary relationship. In analyzing model genomes of radioresistant prokaryotes ( and
and  ), this study assesses the evolutionary itinerary of rough-and-tumble route on which their DNA was assaulted. Together with the examined literature, our analyses suggest the following elements. (1) Based on statistics of ORFs (Open Reading Frames) similarity relationships, we support the hypothesis that radiation resistance is a unique molecular reflection of the early Earth resilience and desiccation tolerance is a mark of cells that colonized land during the Archaean epoch. (2) Using unconventional methods, including oligonucleotide frequencies, numerous compelling data evoke the hypothesis that
), this study assesses the evolutionary itinerary of rough-and-tumble route on which their DNA was assaulted. Together with the examined literature, our analyses suggest the following elements. (1) Based on statistics of ORFs (Open Reading Frames) similarity relationships, we support the hypothesis that radiation resistance is a unique molecular reflection of the early Earth resilience and desiccation tolerance is a mark of cells that colonized land during the Archaean epoch. (2) Using unconventional methods, including oligonucleotide frequencies, numerous compelling data evoke the hypothesis that  and
and  progenitor(s) adapted, at least once, to the same environmental pressure, most probably ionizing radiation, through the acquisition of foreign genetic elements from one abnegator or compositionally similar abnegators. In conclusion, we present a model that satisfactorily accounts, from evolutionary perspectives, for DNA damage in radiation-resistant prokaryotes.
progenitor(s) adapted, at least once, to the same environmental pressure, most probably ionizing radiation, through the acquisition of foreign genetic elements from one abnegator or compositionally similar abnegators. In conclusion, we present a model that satisfactorily accounts, from evolutionary perspectives, for DNA damage in radiation-resistant prokaryotes.
Narumi, Issei; Oba, Hirofumi; Sato, Katsuya; Sghaier, H.
no journal, ,
no abstracts in English

Sato, Katsuya; Oba, Hirofumi; Sghaier, H.; Narumi, Issei
no journal, ,
no abstracts in English
 provides insights into ancestral recombinational DNA repair
provides insights into ancestral recombinational DNA repairSghaier, H.; Oba, Hirofumi; Sato, Katsuya; Mitomo, Hiroshi*; Narumi, Issei
no journal, ,
no abstracts in English

Oba, Hirofumi; Sato, Katsuya; Kikuchi, Masahiro; Narumi, Issei; Sghaier, H.*; Yanagisawa, Tadashi*
no journal, ,
no abstracts in English

Oba, Hirofumi; Sato, Katsuya; Sghaier, H.; Narumi, Issei
no journal, ,
no abstracts in English

Oba, Hirofumi; Sghaier, H.; Sato, Katsuya; Narumi, Issei
no journal, ,
no abstracts in English