Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hashimoto, Kei*; Shiwaku, Toru*; Aoki, Hiroyuki; Yokoyama, Hideaki*; Mayumi, Koichi*; Ito, Kozo*
Science Advances (Internet), 9(47), p.eadi8505_1 - eadi8505_8, 2023/11
Times Cited Count:5 Percentile:45.8(Multidisciplinary Sciences)Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Separation and Purification Technology, 308, p.122943_1 - 122943_7, 2023/03
Times Cited Count:2 Percentile:22.61(Engineering, Chemical)HNO leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO
leaching is used in recycling Pd metal from spent products that primarily contain Ag, and most Pd residues are separated from solutions containing Ag(I). However, a small amount of Pd(II) often remains in these Ag(I) solutions. Therefore, the separation of Pd(II) and Ag(I) in HNO solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO
solutions is essential to promote efficient Pd recycling. In this study, the separation of Pd(II) and Ag(I) in HNO solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO
solutions was investigated using four N-donor-type adsorbents functionalized with amine (R-Amine), iminodiacetic acid (R-IDA), pyridine (R-Py), or bis-picolylamine (R-BPA). R-Amine, R-IDA, and R-Py selectively adsorbed Pd(II) over Ag(I), Cu(II), Ni(II), and Fe(III) from HNO solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,
solutions (0.3-7 M), but R-Amine exhibited a lower Pd adsorption efficiency. In contrast,  90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO
90% of Pd(II), Ag(I), and Cu(II) were adsorbed by R-BPA over the entire range of HNO concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
concentrations. Structural analyses of the adsorbed metal ions using Fourier transform infrared spectroscopy and extended X-ray absorption fine structure spectroscopy revealed the separation mechanisms of the N-donor-type adsorbents. Pd(II) adsorption on R-IDA, R-Py, and R-BPA occurred via Pd(II) coordination of the functional groups (iminodiacetic acid, pyridine, and bis-picolylamine, respectively), whereas that on R-Amine occurred via anion exchange of NO
 with [Pd(NO
with [Pd(NO )
) ]
] . The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
. The coordinative adsorption mechanisms resulted in the higher Pd(II) adsorption behaviors of R-IDA, R-Py, and R-BPA. HCl (5.0 M) and thiourea (0.1 M) eluents desorbed 83% of Pd(II) from R-IDA and 95% from R-Py, respectively. R-Py was the most effective Pd(II) adsorbent based on adsorption selectivity and desorption efficiency.
Suzuki, Tomoya*; Otsubo, Ukyo*; Ogata, Takeshi*; Shiwaku, Hideaki; Kobayashi, Toru; Yaita, Tsuyoshi; Matsuoka, Mitsuaki*; Murayama, Norihiro*; Narita, Hirokazu*
Dalton Transactions (Internet), 50(33), p.11390 - 11397, 2021/09
Times Cited Count:2 Percentile:20.72(Chemistry, Inorganic & Nuclear)no abstracts in English
Sugiura, Yuki; Tomura, Tsutomu*; Ishidera, Takamitsu; Doi, Reisuke; Francisco, P. C. M.; Shiwaku, Hideaki; Kobayashi, Toru; Matsumura, Daiju; Takahashi, Yoshio*; Tachi, Yukio
Journal of Radioanalytical and Nuclear Chemistry, 324(2), p.615 - 622, 2020/05
Times Cited Count:4 Percentile:44.4(Chemistry, Analytical)Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Analytical Sciences, 35(12), p.1353 - 1360, 2019/12
Times Cited Count:4 Percentile:12.17(Chemistry, Analytical)no abstracts in English
 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:13.73(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
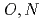 -hetero donor ligand BIZA
-hetero donor ligand BIZAKobayashi, Toru; Akutsu, Kazuhiro*; Nakase, Masahiko*; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation Science and Technology, 54(13), p.2077 - 2083, 2019/02
Times Cited Count:5 Percentile:22.13(Chemistry, Multidisciplinary)Development of separation reagent, which can efficiently separate lanthanides, is of increasing importance because it concerns establishment of purification and recycle techniques rare-earth elements. In our research group, several hetero donor type ligands, which have both oxygen and heterocyclic nitrogen atoms as donor atoms, were developed and revealed that this type of ligands show the selective complexation with specific lanthanide. In this paper, we will discuss the lanthaides complexation properties of the hetero donor type ligand containing benzimidazole group as nitrogen donor ( -methyl-
-methyl- -phenyl-2-(1
-phenyl-2-(1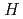 -benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
-benzimidazol-2-yl)-pyridine-6-carboxamide) based on structural investigation by crystallography.
Kobayashi, Toru; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
Progress in Nuclear Science and Technology (Internet), 5, p.74 - 77, 2018/11
Development of extractant, which can efficiently separate actinides, is of increasing importance because it concerns establishment and simplification of separation techniques in nuclear fuel cycle and decontamination of radioactive wastes. In this study, the complexation properties of N-alkyl-N-phenyl-1,10-phenanthroline-2-carboxamide (PTA) with trivalent lanthanides (Ln ) were investigated based on structural analysis by using X-ray crystallography and extended X-ray absorption fine structure (EXAFS) methods. As a result, it is revealed that two PTA molecules coordinate with Ln
) were investigated based on structural analysis by using X-ray crystallography and extended X-ray absorption fine structure (EXAFS) methods. As a result, it is revealed that two PTA molecules coordinate with Ln as tridentate ligand via two nitrogen in phenanthroline moiety and one oxygen in amide moiety in both crystal and solution states. The slight difference in coordination bond distances are observed between Eu
as tridentate ligand via two nitrogen in phenanthroline moiety and one oxygen in amide moiety in both crystal and solution states. The slight difference in coordination bond distances are observed between Eu and Nd
and Nd complexes, this difference corresponds to the difference in ionic radius between Nd
complexes, this difference corresponds to the difference in ionic radius between Nd and Eu
and Eu . This result indicates slight difference in ionic radii of Ln
. This result indicates slight difference in ionic radii of Ln hardly affects coordination properties of PTA.
hardly affects coordination properties of PTA.
Nakase, Masahiko*; Kobayashi, Toru; Shiwaku, Hideaki; Kawamura, Takuya*; Takeshita, Kenji*; Yamamura, Tomoo*; Yaita, Tsuyoshi
Progress in Nuclear Science and Technology (Internet), 5, p.56 - 60, 2018/11
Gel/liquid extraction was investigated to achieve selective separation of trivalent actinides over lanthanides. In this study, thermosensitive hydrogel including N,N,N',N'-tetraallylpyridine-2,6-dicarboxamine (PDA) was synthesized, and its complexation with lanthanide was investigated by extended X-ray absorption fine structure experiments at SPring-8. The radial structure functions (RSF) of solution with gels with and without ligands at different temperatures showed slight differences. As the temperature increased, the RSF decreased owing to the increase in thermal vibration, but PDA-gel showed a shift in the first peak toward the shorter direction. This shift was attributed to the change in stoichiometry of PDA-Ln(III) complexes or coordination number of water molecules affected by the change of conformation of polymer chains and the hydrophilic/hydrophobic properties in the hydrogel.
Suzuki, Tomoya*; Ogata, Takeshi*; Tanaka, Mikiya*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi; Narita, Hirokazu*
Metals, 8(7), p.558_1 - 558_10, 2018/07
Times Cited Count:13 Percentile:53.43(Materials Science, Multidisciplinary)The refining of platinum group metals is based mainly on solvent extraction methods, whereas Ru is selectively recovered by distillation as RuO . Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl
. Replacement of distillation byextraction is expected to simplify the purification process. To develop an effective extraction system for Ru, we analyzed the Ru species in HCl with UV-Vis and EXAFS spectroscopies, and we examined the properties of Ru extracted with N-2-ethylhexyl-bis(N-di-2-ethylhexyl-ethylamide) amine (EHBAA). EXAFS and UV-Vis spectra of Ru in HCl solutions revealed that the predominant Ru species in 0.5-10 M HCl solutions changed from [RuCl (H
(H O)
O) ]
] to [RuCl
to [RuCl ]
]
 with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
with the HCl concentration. The extraction percentages of Ru in the EHBAA system increased with increasing HCl concentration, reached 80% at [HCl] = 5 M, and decreased athigher HCl concentrations. EXAFS analysis of the extracted complex indicated that the Ru
 had 5 Cl
had 5 Cl and 1 H
and 1 H O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl
O in its inner coordination sphere. The similarity of the dependence on HCl concentrations of the extraction in the EHBAA system and the distribution profile of [RuCl (H
(H O)]
O)]
 on [RuCl
on [RuCl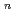 (H
(H O)
O)
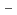
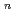 ]
]

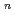 suggested that the EHBAA extracted the pentachlorido species.
suggested that the EHBAA extracted the pentachlorido species.
Yoshigoe, Akitaka; Shiwaku, Hideaki; Kobayashi, Toru; Shimoyama, Iwao; Matsumura, Daiju; Tsuji, Takuya; Nishihata, Yasuo; Kogure, Toshihiro*; Okochi, Takuo*; Yasui, Akira*; et al.
Applied Physics Letters, 112(2), p.021603_1 - 021603_5, 2018/01
Times Cited Count:6 Percentile:29.69(Physics, Applied)A synchrotron radiation photoemission electron microscope (SR-PEEM) was applied to demonstrate pinpoint analysis of micrometer-sized weathered biotite clay particles with artificially adsorbed cesium (Cs) atoms. Despite the insulating properties of the clay, we observed the spatial distributions of constituent elements (Si, Al, Cs, Mg, Fe) without charging issues. We found that Cs atoms were likely to be adsorbed evenly over the entire particle. Spatially-resolved X-ray absorption spectra (XAS) of the Cs M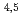 -edge region showed Cs to be present in a monocation state (Cs
-edge region showed Cs to be present in a monocation state (Cs ). Further pinpoint XAS measurements were also performed at the Fe L
). Further pinpoint XAS measurements were also performed at the Fe L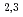 -edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
-edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
Okamoto, Yoshihiro; Osugi, Takeshi; Akabori, Mitsuo; Kobayashi, Toru; Shiwaku, Hideaki
Journal of Molecular Liquids, 232, p.285 - 289, 2017/04
Times Cited Count:3 Percentile:12.07(Chemistry, Physical)High energy XAFS measurement using cerium K-edge was performed to study the chemical state of cerium in high-temperature molten slag (SiO -CaO-Fe
-CaO-Fe O
O -CeO
-CeO ). It was found from the change in the nearest Ce-O distance obtained from EXAFS analysis and the energetic shift of the white line peak observed in XANES analysis that oxidation state of cerium was tetravalent in the molten state and trivalent in solid state. The Debye-Waller factor of the nearest Ce-O pair in solid slag was very large even at room temperature, and the change in its value upon heating and melting was very small. This result suggests that cerium is highly disordered and stable in solid slag.
). It was found from the change in the nearest Ce-O distance obtained from EXAFS analysis and the energetic shift of the white line peak observed in XANES analysis that oxidation state of cerium was tetravalent in the molten state and trivalent in solid state. The Debye-Waller factor of the nearest Ce-O pair in solid slag was very large even at room temperature, and the change in its value upon heating and melting was very small. This result suggests that cerium is highly disordered and stable in solid slag.
Nakase, Masahiko*; Takeshita, Kenji*; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Separation Science and Technology, 50, p.2832 - 2835, 2015/05
Times Cited Count:1 Percentile:4.99(Chemistry, Multidisciplinary)Separation of trivalent lanthanides (Ln) and trivalent actinides (An) is important task in reprocessing high-level liquid waste. In this study, the relationship between structures of complexes of 2,2'-bipyridyl (Bpy) and Ln and coordination strengths were investigated by X-ray diffraction and UV titration. With the decrease in ionic radius from Nd to Er, the N(Bpy)-Ln distance and N(Bpy)-Ln-N(Bpy) angle decreased, the dihedral angle of the Bpy pyridines increased, and the stability constant increased. This information about structure and coordination strength is important for designing better ligands for separating Ln and An ions.
Awual, M. R.; Kobayashi, Toru; Shiwaku, Hideaki; Miyazaki, Yuji; Motokawa, Ryuhei; Suzuki, Shinichi; Okamoto, Yoshihiro; Yaita, Tsuyoshi
Chemical Engineering Journal, 225, p.558 - 566, 2013/06
Times Cited Count:198 Percentile:98.67(Engineering, Environmental)Awual, M. R.; Kobayashi, Toru; Miyazaki, Yuji; Motokawa, Ryuhei; Shiwaku, Hideaki; Suzuki, Shinichi; Okamoto, Yoshihiro; Yaita, Tsuyoshi
Journal of Hazardous Materials, 252-253, p.313 - 320, 2013/05
Times Cited Count:185 Percentile:97.62(Engineering, Environmental)Akutsu, Kazuhiro*; Suzuki, Shinichi; Kobayashi, Toru; Shiwaku, Hideaki; Okamoto, Yoshihiro; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 20, p.105 - 114, 2013/00
 -methyl-
-methyl- -phenyl-1,10-phenanthroline-2-carboxamide
-phenyl-1,10-phenanthroline-2-carboxamideKobayashi, Toru; Suzuki, Shinichi; Shiwaku, Hideaki; Yaita, Tsuyoshi
X-ray Structure Analysis Online (Internet), 28(10), p.77 - 78, 2012/10
Kobayashi, Toru; Yaita, Tsuyoshi; Suzuki, Shinichi; Shiwaku, Hideaki; Okamoto, Yoshihiro; Akutsu, Kazuhiro*; Nakano, Yoshiharu*; Fujii, Yuki*
Separation Science and Technology, 45(16), p.2431 - 2436, 2010/11
Times Cited Count:35 Percentile:71.06(Chemistry, Multidisciplinary)Numakura, Masahiko; Yaita, Tsuyoshi; Shiwaku, Hideaki; Suzuki, Shinichi; Kobayashi, Toru*; Akutsu, Kazuhiro; Madden, P. A.*; Okamoto, Yoshihiro
JAEA-Research 2009-003, 26 Pages, 2009/04
We investigated the mixing behavior of molten TbCl in LiCl-KCl eutectuic melt by MD simulation. Also, simulation of molten YCl
in LiCl-KCl eutectuic melt by MD simulation. Also, simulation of molten YCl and LaCl
and LaCl systems were performed to elucidate the difference of the structural change by the difference in cation size (Y
systems were performed to elucidate the difference of the structural change by the difference in cation size (Y
 Tb
Tb
 La
La ). The coordination number of the molten pure YCl
). The coordination number of the molten pure YCl , TbCl
, TbCl and LaCl
and LaCl are mainly 6, 7 and 8 respectively. We consider that, in the molten pure state, the coordination number is influenced by the cation size. However, independent of cation size, the molten MCl
are mainly 6, 7 and 8 respectively. We consider that, in the molten pure state, the coordination number is influenced by the cation size. However, independent of cation size, the molten MCl tend to be formed the stable 6-fold structure by the mixing with LiCl-KCl eutectic. And, MD simulations on MCl
tend to be formed the stable 6-fold structure by the mixing with LiCl-KCl eutectic. And, MD simulations on MCl -LiCl and MCl
-LiCl and MCl -KCl systems revealed that the mixing effect was different between LiCl and KCl.
-KCl systems revealed that the mixing effect was different between LiCl and KCl.
Shiwaku, Hideaki; Yaita, Tsuyoshi; Kobayashi, Toru; Numakura, Masahiko; Suzuki, Shinichi; Okamoto, Yoshihiro
Proceedings of 4th Workshop on Speciation, Techniques, and Facilities for Radioactive Materials at Synchrotron Light Sources (Actinide-XAS-2006), p.301 - 306, 2007/05
no abstracts in English