Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
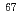 Cu produced by
Cu produced by 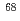 Zn(
Zn( )
)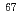 Cu to biodistribution study in tumor-bearing mice
Cu to biodistribution study in tumor-bearing miceSugo, Yumi*; Hashimoto, Kazuyuki*; Kawabata, Masako*; Saeki, Hideya*; Sato, Shunichi*; Tsukada, Kazuaki; Nagai, Yasuki*
Journal of the Physical Society of Japan, 86(2), p.023201_1 - 023201_3, 2017/02
Times Cited Count:15 Percentile:66.37(Physics, Multidisciplinary)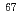 Cu produced by the
Cu produced by the 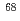 Zn(
Zn(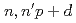 )
) Cu reaction was used for the first time to determine the biodistribution of
Cu reaction was used for the first time to determine the biodistribution of 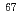 CuCl
CuCl in colorectal tumor-bearing mice. High uptake of
in colorectal tumor-bearing mice. High uptake of 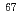 Cu was observed in the tumor as well as in the liver and kidney which are the major organs for copper metabolism. The result showing
Cu was observed in the tumor as well as in the liver and kidney which are the major organs for copper metabolism. The result showing 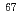 Cu accumulation in the tumor suggests that
Cu accumulation in the tumor suggests that 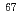 CuCl
CuCl can be a potential radionuclide agent for cancer radiotherapy. It would also encourage further studies on the therapeutic effect in small animals using an increased dose of
can be a potential radionuclide agent for cancer radiotherapy. It would also encourage further studies on the therapeutic effect in small animals using an increased dose of 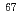 Cu produced by the
Cu produced by the 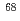 Zn(
Zn(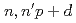 )
)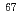 Cu reaction using intense neutrons available at present.
Cu reaction using intense neutrons available at present.
 evaluation of F(
evaluation of F( -
- I)KCCYSL for targeting HER2
I)KCCYSL for targeting HER2Sasaki, Ichiro; Watanabe, Shigeki; Ohshima, Yasuhiro; Sugo, Yumi; Yamada, Keiichi*; Hanaoka, Hirofumi*; Ishioka, Noriko
Peptide Science 2015, p.243 - 246, 2016/03
Mori, Masanobu*; Sagara, Katsuya*; Arai, Kaori*; Nakatani, Nobutake*; Ohira, Shinichi*; Toda, Kei*; Itabashi, Hideyuki*; Kozaki, Daisuke*; Sugo, Yumi; Watanabe, Shigeki; et al.
Journal of Chromatography A, 1431, p.131 - 137, 2016/01
Times Cited Count:10 Percentile:38.10(Biochemical Research Methods)Sasaki, Yuji; Sugo, Yumi; Morita, Keisuke; Nash, K.*; Nash, K. L.*
Solvent Extraction and Ion Exchange, 33(7), p.625 - 641, 2015/10
Times Cited Count:112 Percentile:91.79(Chemistry, Multidisciplinary)The effect of alkyl substituents at amidic N atoms in diglycolamide (DGA) compounds on solvent extraction was investigated. The solubility in water and n-dodecane, loading capacity, and distribution ratio (D) of lanthanides and actinides for various DGA compounds were monitored. DGA derivatives with short alkyl chains, for example methyl and ethyl groups, are highly water soluble, while DGA derivatives with long alkyl chains are highly soluble in n-dodecane. The loading capacity correlats with their alkyl chain lengths. The results of masking effect and solubility tests indicate that TEDGA(tetraethyl-diglycolamide) is the best actinide masking agent among the water-soluble DGA derivatives. Actinide extractions were performed using ten DGA compounds in six diluents (nitrobenzene, 1,2-dichloroethane, 1-octanol, chloroform, toluene, and n-dodecane), in which it was observed that DGA derivatives with shorter alkyl chains show higher D values.
Sasaki, Yuji; Saeki, Morihisa; Sugo, Yumi; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Ohashi, Akira*
Solvent Extraction Research and Development, Japan, 22(1), p.37 - 45, 2015/05
Times Cited Count:15 Percentile:41.95(Chemistry, Multidisciplinary)An extractant, methylimino-bis- -dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis-
-dioctylacetamide (MIDOA), was used for the extraction of soft acid metals. It was found that MIDOA can extract not only Cr(VI), Mo(VI), W(VI), Tc(VII) and Re(VII), whose metals can form the oxonium anions due to their high oxidation states, but also other metal cations, like Nb(V), Ta(V) and Pd(II). Analogous compounds, imino-bis- -dioctylacetamide (IDOA) and methylimino-bis-
-dioctylacetamide (IDOA) and methylimino-bis- -di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower
-di-2-ethylhexylacetamide (MIDEHA), are synthesized and compared for their extractability. It is clear that these extractants have almost same or lower  values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA (
values than those for MIDOA. In order to examine the effect on extractability with different donor atoms, TODGA ( -tetraoctyl-diglycolamide) and TDGA (
-tetraoctyl-diglycolamide) and TDGA ( -tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N
-tetraoctyl-tyiodiglycolamide) having oxygen and sulfur donor are employed. The comparison of their extractabilities suggests that the trend of Pd and Re extraction is N  S
S  O and N
O and N  O
O  S, respectively.
S, respectively.
 evaluation of
evaluation of  Cu-labeled peptide for tumor imaging
Cu-labeled peptide for tumor imagingSugo, Yumi; Sasaki, Ichiro; Watanabe, Shigeki; Ohshima, Yasuhiro; Ishioka, Noriko
JAEA-Review 2014-050, JAEA Takasaki Annual Report 2013, P. 100, 2015/03
 studies on cellular binding and stability of
studies on cellular binding and stability of  Cu-labeled peptide for tumor imaging
Cu-labeled peptide for tumor imagingSugo, Yumi; Ohshima, Yasuhiro; Sasaki, Ichiro; Ishioka, Noriko
Peptide Science 2014, p.303 - 306, 2015/03
Sasaki, Ichiro; Hanaoka, Hirofumi*; Yamada, Keiichi*; Watanabe, Shigeki; Sugo, Yumi; Ohshima, Yasuhiro; Suzuki, Hiroyuki; Ishioka, Noriko
Peptide Science 2014, p.257 - 260, 2015/03
 -radiation effect on solvent extraction of minor actinide
-radiation effect on solvent extraction of minor actinideSugo, Yumi; Sasaki, Yuji; Taguchi, Mitsumasa; Ishioka, Noriko
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1381 - 1384, 2015/02
Times Cited Count:5 Percentile:36.21(Chemistry, Analytical)Sasaki, Yuji; Kitatsuji, Yoshihiro; Sugo, Yumi; Tsubata, Yasuhiro; Suzuki, Tomoya; Kimura, Takaumi; Morita, Yasuji
Proceedings of 20th International Solvent Extraction Conference (ISEC 2014), p.431 - 435, 2014/09
The eight amide extractants including podand-types, which introduce oxygen, nitrogen and sulfur donor atoms in their central frame, are synthesized and their D values for actinide extraction are compared. The central frame affect greatly on their extractability and it is clear that diglycolamide (DGA) has the highest extractability among extractants used here. In addition, DGA having the different length of alkyl chain or phenyl groups attached to amidic N atoms are obtained and the effect of these substituents is studied. Due to the steric hindrance or hydrogen bond, DGA with long and branched alkyl chains, phenyl group, and proton give relatively low D values.
Sasaki, Yuji; Tsubata, Yasuhiro; Kitatsuji, Yoshihiro; Sugo, Yumi; Shirasu, Noriko; Ikeda, Yasuhisa*; Kawasaki, Takeshi*; Suzuki, Tomoya*; Mimura, Hitoshi*; Usuda, Shigekazu*; et al.
JAEA-Research 2014-008, 220 Pages, 2014/06
The researches on Development of mutual separation technology of minor actinides by the novel hydrophilic and lipophilic diamide compounds, entrusted to Japan Atomic Energy Agency by the Ministry of Education, Culture, Sports, Science and Technology of Japan, from 2010 to 2012 are summarized. This project was composed of three themes, those are (1) Development of total recovery of MA+Ln: basic researches for new extractant, DOODA, (2) Development of mutual separation of Am/Cm/Ln: basic researches of Ln-complex, solvent extraction, and extraction chromatography, and (3) Evaluation of separation technique: process simulation. For topic (1), we summarized the information on characteristic of DOODA extractant. For topic (2), we summarized the information on structures of Ln-complexes, solvent extraction and chromatography. For topic (3), we summarized the information on conditions of mixer-settler and evaluation of each fraction separated.
 -ray irradiation
-ray irradiationArisaka, Makoto; Watanabe, Masayuki; Sugo, Yumi; Kobayashi, Kumiko*; Kanao, Osamu*; Kimura, Takaumi
Journal of Nuclear Science and Technology, 51(4), p.457 - 464, 2014/04
Times Cited Count:3 Percentile:21.88(Nuclear Science & Technology)Toward the development of a practical separation method for trivalent actinides and lanthanides, we used extraction chromatography with alkyl-pyridinedicarboxyamides as the extractant. The results confirmed that the performance degradation of the adsorbent caused by contact with HNO and/or irradiation with
and/or irradiation with  rays would be very small during the operation of column chromatography. The optimal conditions for the column separation were also determined: eluent, 5M HNO
rays would be very small during the operation of column chromatography. The optimal conditions for the column separation were also determined: eluent, 5M HNO ; flow rate, 0.1 mL/min.
; flow rate, 0.1 mL/min.
Sugo, Yumi; Sasaki, Yuji; Ishioka, Noriko
JAEA-Review 2013-059, JAEA Takasaki Annual Report 2012, P. 17, 2014/03
Sasaki, Ichiro; Yamada, Keiichi*; Watanabe, Shigeki; Hanaoka, Hirofumi*; Sugo, Yumi; Oku, Hiroyuki*; Ishioka, Noriko
JAEA-Review 2013-059, JAEA Takasaki Annual Report 2012, P. 96, 2014/03
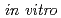 Evaluation of
Evaluation of  Cu-labeled peptide for tumor imaging
Cu-labeled peptide for tumor imagingSugo, Yumi; Sasaki, Ichiro; Watanabe, Shigeki; Ohshima, Yasuhiro; Ishioka, Noriko
Peptide Science 2013, p.355 - 358, 2014/03
Sasaki, Yuji; Tsubata, Yasuhiro; Kitatsuji, Yoshihiro; Sugo, Yumi; Shirasu, Noriko; Morita, Yasuji
Proceedings of International Nuclear Fuel Cycle Conference; Nuclear Energy at a Crossroads (GLOBAL 2013) (CD-ROM), p.1079 - 1082, 2013/09
Mutual separation of Am, Cm and lanthanides (Ln) is important to develop the partitioning process of high-level radioactive liquid waste, because the application to their different disposal methods are advantageous. Namely, Am is studied for transmutation due to the reduction of long half-life radionuclides, Cm should be kept in interim storage in order to reduce the calorific value, and Ln should be present in the vitrified radioactive waste toward the geological disposal. However, this mutual separation method is difficult to establish because they have very similar chemical behavior, same oxidation state (III) and similar ionic radii. The development of their mutual separation is termed as the challenging study. In order to obtain the satisfactory results, the property of extractant requires the differentiation of actinide (An) from Ln, high preferability to different ionic radii between Am and Cm, and high extractability to hard acids. Therefore, the extractant have to include both N atom, whose soft donor has high selectivity between An and Ln, and O atoms for the strong extractability to An. The new extractant, NTAamide (N,N,N',N',N'',N''-hexaoctyl-nitrirotriacetamide) is a triamide having N donor at the center of backbone, then NTAamide has hybrid performance of complexation to metals by soft N and three hard amidic O atoms. It is clear that NTAamide can extract trivalent An at diluted HNO with small D(Ln), the separation of An from Ln can be carried out at that condition. The SF of Am/Cm by NTAamide is approximate 1.8, which is not so high to separate each other. The combination of NTAamide of extractant and TEDGA (N,N,N',N'-tetraethyl-diglycolamide) as a masking agent in the aqueous phase shows very high SF(Am/Cm) of maximal 6.5. It is obvious that NTAamide is a promising extractant to achieve the mutual separation among Am/Cm/Ln.
with small D(Ln), the separation of An from Ln can be carried out at that condition. The SF of Am/Cm by NTAamide is approximate 1.8, which is not so high to separate each other. The combination of NTAamide of extractant and TEDGA (N,N,N',N'-tetraethyl-diglycolamide) as a masking agent in the aqueous phase shows very high SF(Am/Cm) of maximal 6.5. It is obvious that NTAamide is a promising extractant to achieve the mutual separation among Am/Cm/Ln.
Sasaki, Ichiro; Yamada, Keiichi*; Watanabe, Shigeki; Hanaoka, Hirofumi*; Sugo, Yumi; Oku, Hiroyuki*; Ishioka, Noriko
Peptide Science 2013, p.157 - 160, 2013/03
Sugo, Yumi; Taguchi, Mitsumasa; Sasaki, Yuji; Morita, Yasuji; Ishioka, Noriko
JAEA-Review 2012-046, JAEA Takasaki Annual Report 2011, P. 18, 2013/01
 -dodecane
-dodecaneSugo, Yumi; Taguchi, Mitsumasa; Sasaki, Yuji; Morita, Yasuji
JAEA-Review 2011-043, JAEA Takasaki Annual Report 2010, P. 18, 2012/01
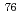 Br]bromo-
Br]bromo- -methyl-L-tyrosine, a novel tyrosine analog for PET imaging of tumors
-methyl-L-tyrosine, a novel tyrosine analog for PET imaging of tumorsOhshima, Yasuhiro; Hanaoka, Hirofumi*; Watanabe, Shigeki; Sugo, Yumi; Watanabe, Satoshi; Tominaga, Hideyuki*; Oriuchi, Noboru*; Endo, Keigo*; Ishioka, Noriko
JAEA-Review 2011-043, JAEA Takasaki Annual Report 2010, P. 91, 2012/01