Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagae, Daisuke*; Abe, Yasushi*; Okada, Shunsuke*; Omika, Shuichiro*; Wakayama, Kiyoshi*; Hosoi, Shun*; Suzuki, Shinji*; Moriguchi, Tetsuro*; Amano, Masamichi*; Kamioka, Daiki*; et al.
Nuclear Instruments and Methods in Physics Research A, 986, p.164713_1 - 164713_7, 2021/01
Times Cited Count:5 Percentile:65.59(Instruments & Instrumentation)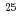 F from
F from  O
O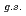 ?
?Tang, T. L.*; Uesaka, Tomohiro*; Kawase, Shoichiro; Beaumel, D.*; Dozono, Masanori*; Fujii, Toshihiko*; Fukuda, Naoki*; Fukunaga, Taku*; Galindo-Uribarri, A.*; Hwang, S. H.*; et al.
Physical Review Letters, 124(21), p.212502_1 - 212502_6, 2020/05
Times Cited Count:14 Percentile:74.18(Physics, Multidisciplinary)The structure of a neutron-rich 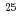 F nucleus is investigated by a quasifree (
F nucleus is investigated by a quasifree (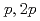 ) knockout reaction. The sum of spectroscopic factors of
) knockout reaction. The sum of spectroscopic factors of 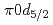 orbital is found to be 1.0
orbital is found to be 1.0  0.3. The result shows that the
0.3. The result shows that the  O core of
O core of 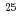 F nucleus significantly differs from a free
F nucleus significantly differs from a free  O nucleus, and the core consists of
O nucleus, and the core consists of  35%
35%  O
O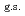 , and
, and  65% excited
65% excited  O. The result shows that the
O. The result shows that the  O core of
O core of 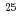 F nucleus significantly differs from a free
F nucleus significantly differs from a free  O nucleus. The result may infer that the addition of the
O nucleus. The result may infer that the addition of the 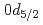 proton considerably changes the neutron structure in
proton considerably changes the neutron structure in 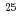 F from that in
F from that in  O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
Yanagisawa, Tsutomu*; Usami, Shin; Maeda, Seiichiro
Genshiryoku Nenkan 2018, p.90 - 95, 2017/10
no abstracts in English
Yanagisawa, Ichiro*; Katsurai, Kiyomichi*; Izumi, Jun*; Saigusa, Moriyuki*; *; *; *; Ueta, Shinzo*
JNC TJ8400 2000-038, 202 Pages, 2000/02
(1)Sodalite and tourmaline are natural-occurring minerals, which can contain halide in their aluminosilicate lattices. Therefore, these materials have possibilities of immobilization of I-129. In this study, solubility measurements for these materials were carried out. It was confirmed from dissolution behaviors obtained for some kinds of sodalite and calculated results of solubilities based on thermodynamic data that dissolution of sodalite to aqueous solution could be limited by its solubility. Solubility of sodalite had tendencies of "synthesized one  natural one" and "chloride
natural one" and "chloride  iodide". Immobilized iodine in sodalite crystalline lattice was not replaced by chlolide ion in the solution. It was indicated that tourmaline has a possibility as a waste material containing I-129 from comparison of dissolution behavior of element with sodalite. (2)Leaching property of a multi-layered waste-form, that is composed of (1)iodine bearing material (zeolite), (2)coating layer (silica and apatite) and 3)low solubility matirx (apatite), was studied under reducing condition. The following results were obtained by the leaching experiments: (1)The coating layer of hidroxyapatite can reduce the iodine leaching rate by 4 order compared with that of bare iodine bearing material. (2)Ca and P concentration after one-month dipping reached the solubility estimated by the theoretical calculation using PHREEQE code. As a conclusion, it was indicated that this waste-form concept has potential to show low leaching rate.
iodide". Immobilized iodine in sodalite crystalline lattice was not replaced by chlolide ion in the solution. It was indicated that tourmaline has a possibility as a waste material containing I-129 from comparison of dissolution behavior of element with sodalite. (2)Leaching property of a multi-layered waste-form, that is composed of (1)iodine bearing material (zeolite), (2)coating layer (silica and apatite) and 3)low solubility matirx (apatite), was studied under reducing condition. The following results were obtained by the leaching experiments: (1)The coating layer of hidroxyapatite can reduce the iodine leaching rate by 4 order compared with that of bare iodine bearing material. (2)Ca and P concentration after one-month dipping reached the solubility estimated by the theoretical calculation using PHREEQE code. As a conclusion, it was indicated that this waste-form concept has potential to show low leaching rate.
Yanagisawa, Ichiro*; Katsurai, Kiyomichi*; Izumi, Jun*; Saigusa, Moriyuki*; Kitao, Hideo*; Tsuzuki, Yasuo*; Neyama, Atsushi*; Kato, Hiroyasu*; Nakazawa, Toshiyuki*; Okada, Kenichi*
JNC TJ8400 2000-037, 61 Pages, 2000/02
no abstracts in English
Yanagisawa, Ichiro*; Katsurai, Kiyomichi*; Mukai, Satoru*; *
JNC TJ8400 2000-028, 224 Pages, 2000/02
In this study, in-diffusion experiment was conducted under reducing condition to measure apparent diffusion coefficients and distribution coefficients of Uranium and Neptunium for bentonite materials as engineered barrier. The summary of this study is as follows: (1)Apparent diffusion coefficients of U and Np under reducing condition were one or two orders magnitude lower than those of the results obtained in the previous studies. From this result, the valence of U and Np was estimated to be +4 charge during diffusion experiment. (2)Distribution coefficients of U and Np for the system of equilibriated water with bentonite at density of 1.6 g/cm were a little lower than those for the other synthetic groundwater system. (3)Distribution coefficients of Cs and Sr decreased as ion strength of pore water in bentonite increased. Distribution coefficient of Sr increased for alternation of bentonite. (4)Distribution coefficients of Ni, Sn, Zr and Nb were little difference for any type of bentonite or synthetic groundwater. (5)Distribution coefficients or Am for Na-type of bentonite was higher than that of Ca-type of bentonite. But the difference of distribution coefficients for any synthetic groundwater was small. (6)Data base of apparent diffusion coefficients and distribution coefficients was renewed.
were a little lower than those for the other synthetic groundwater system. (3)Distribution coefficients of Cs and Sr decreased as ion strength of pore water in bentonite increased. Distribution coefficient of Sr increased for alternation of bentonite. (4)Distribution coefficients of Ni, Sn, Zr and Nb were little difference for any type of bentonite or synthetic groundwater. (5)Distribution coefficients or Am for Na-type of bentonite was higher than that of Ca-type of bentonite. But the difference of distribution coefficients for any synthetic groundwater was small. (6)Data base of apparent diffusion coefficients and distribution coefficients was renewed.
Yanagisawa, Ichiro*; Katsurai, Kiyomichi*; Mukai, Satoru*; *
JNC TJ8400 2000-027, 28 Pages, 2000/02
no abstracts in English
Tachikawa, Hirokazu*; Kitao, Hideo*; Katsurai, Kiyomichi*; Yanagisawa, Ichiro*; Shibata, Masahiro; Suyama, Tadahiro*; Yui, Mikazu
JNC TN8400 99-068, 108 Pages, 1999/11
In an evaluation of high level waste (HLW) repository performance, Se-79 is one of important elements to be analyzed. Selenium solubility and solubility limiting solid phase is not clear. Then, we performed solubility measurement tests from over saturation direction under reducing conditions considering the repository conditions in deep underground. In some cases, bentonite (Kunigel V1) or pyrite coexisted in the experimental system to simulate the repository conditions. Se bearing solids were determined by XRD analysis, and solubility limiting solid phase was discussed. FeSe (Ferroselite) and Se (hexagonal) were identified in the simple condition test, in which Fe(II) solution and Se solution were mixed. SeS was also identified when S(-II) solution was added. The Se concentrations in aqueous phase were approximately 10
(Ferroselite) and Se (hexagonal) were identified in the simple condition test, in which Fe(II) solution and Se solution were mixed. SeS was also identified when S(-II) solution was added. The Se concentrations in aqueous phase were approximately 10 mol/l at neutral pH and approximately 10
mol/l at neutral pH and approximately 10 mol/l at pH9 in the bentonite coexisting tests and pyrite coexisting tests. The solid phases identified in the pyrite coexisting tests were mainly Se(hexagonal) and FeSe
mol/l at pH9 in the bentonite coexisting tests and pyrite coexisting tests. The solid phases identified in the pyrite coexisting tests were mainly Se(hexagonal) and FeSe (Ferroselite) in one of the samples. Further, the possibility of Fe(S,Se) solid solution formation was presumed on the pyrite surface dipping in the test solutions. In addition, we performed another selenium solubility measurement to accelerate the transformation of Se bearing solid phase at elevated temperature (80
(Ferroselite) in one of the samples. Further, the possibility of Fe(S,Se) solid solution formation was presumed on the pyrite surface dipping in the test solutions. In addition, we performed another selenium solubility measurement to accelerate the transformation of Se bearing solid phase at elevated temperature (80 C). The concentration of Se decreases with time and reached to the detection limit of ICP-MS (4
C). The concentration of Se decreases with time and reached to the detection limit of ICP-MS (4 10
10 mol/1) in 3 months. At first, Se(hexagonal) is dominant in the precipitation, however this solid phase was gradually transformed to Fe-Se solids (FeSe, FeSe
mol/1) in 3 months. At first, Se(hexagonal) is dominant in the precipitation, however this solid phase was gradually transformed to Fe-Se solids (FeSe, FeSe ) with time. Therefore it is strongly suggested that FeSe
) with time. Therefore it is strongly suggested that FeSe which is the thermodynamically most stable phase will be a solubility limiting solid phase under repository conditions in long term. As the experimental system was confirmed as sulfate reducing bacteria free, it should be noted that whole observed reactions ...
which is the thermodynamically most stable phase will be a solubility limiting solid phase under repository conditions in long term. As the experimental system was confirmed as sulfate reducing bacteria free, it should be noted that whole observed reactions ...
Yanagisawa, Ichiro*; Naito, Taisei*; Kitao, Hideo*; Mukai, Satoru*; Doi, Hideo*
JNC TJ8400 99-020, 25 Pages, 1999/02
In this study, apparent diffusion coefficients and distribution coefficient of some nuclides were measured for bentonite materials as engineered barrier. The summary of this study is as follows: (1)Apparent diffusion coefficients of nuclides in compacted bentonite were measured. The data so far were summarized as database of apparent diffusion coefficients and distribution coefficients. (2)In diffusion tests for mixture of Ca-type altered bentonite and analcime, apparent diffusion coefficient of Sr was a little smaller than that for Ca-type altered bentonite. (3)In diffusion tests for Ca-type altered bentonite, apparent diffusion coefficients and distribution coefficients of nuclides showed no significant difference between bentonite-water system and bentonite-cementitious solution systems. (4)In Na-type bentonite-solution with nitrate system, apparent diffusion coefficients of Cs and Sr were smaller than that for other bentonite-solution systems. (5)Apparent diffusion coefficients of tritium showed no significant difference between Ca-type altered bentonite and Na-type bentonite in any solution systems.
Fusaeda, Shigeki*; Yanagisawa, Ichiro*; Kataoka, Shinichi*; Shimada, Takashi*; *; *; *
PNC TJ1216 98-007, 46 Pages, 1998/02
None
Fusaeda, Shigeki*; Yanagisawa, Ichiro*; Kataoka, Shinichi*; Shimada, Takashi*; *; *; *
PNC TJ1216 98-006, 673 Pages, 1998/02
None
Kataoka, Shinichi*; Kitao, Hideo*; Tachikawa, Hirokazu*; Shimada, Takashi*; Maeda, Kazuto*; Nemoto, Kazuaki*; Yanagisawa, Ichiro*
PNC TJ1216 98-002, 676 Pages, 1998/02
None
Muramatsu, Kazuhiro; Murakami, Hiroyuki*; Higashida, Akihiro*; Yanagisawa, Ichiro*
Keisan Kogaku Koenkai Rombunshu, 2(1), p.113 - 116, 1997/05
no abstracts in English
*; Fusaeda, Shigeki*; Kataoka, Shinichi*; Nemoto, Kazuhiko*; Yanagisawa, Ichiro*; Fukui, Hiroshi*; Doi, Motoo*
PNC TJ1216 97-007, 77 Pages, 1997/03
None
*; *; Kataoka, Shinichi*; Nemoto, Kazuhiko*; Yanagisawa, Ichiro*; Fukui, Hiroshi*; Doi, Motoo*
PNC TJ1216 97-006, 202 Pages, 1997/03
None
Mukai, Satoru*; Kitao, Hideo*; Tachikawa, Hirokazu*; Fusaeda, Shigeki*; Yanagisawa, Ichiro*; Doi, Motoo*; *
PNC TJ1216 96-003, 106 Pages, 1996/03
None
Fusaeda, Shigeki*; Yanagisawa, Ichiro*; Uzawa, Masayuki*; *; Kasai, Masao*; Ishihara, Yoshinao*; *
PNC TJ1216 95-002, 191 Pages, 1995/02
None
Yanagisawa, Ichiro*; Fusaeda, Shigeki*; Uzawa, Masayuki*; Kasai, Masao*; Ishihara, Yoshinao*; Ikeda, Yasuhiro*; Ezaki, Masahiro*
PNC TJ1214 94-007, 92 Pages, 1994/03
no abstracts in English
Yanagisawa, Ichiro*; Fusaeda, Shigeki*; Uzawa, Masayuki*; Kasai, Masao*; Ishihara, Yoshinao*; Ikeda, Yasuhiro*; Ezaki, Masahiro*
PNC TJ1214 94-006, 542 Pages, 1994/03
None
Yanagisawa, Kazuaki; ; Horiki, Oichiro; Soyama, Kazuhiko; Ichikawa, Hiroki; Kodaira, Tsuneo
Journal of Nuclear Science and Technology, 30(8), p.741 - 751, 1993/08
Times Cited Count:4 Percentile:44.87(Nuclear Science & Technology)no abstracts in English