Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamaguchi, Akiko; Kurihara, Yuichi*; Nagata, Kojiro*; Tanaka, Kazuya; Higaki, Shogo*; Kobayashi, Toru; Tanida, Hajime; Ohara, Yoshiyuki*; Yokoyama, Keiichi; Yaita, Tsuyoshi; et al.
Journal of Colloid and Interface Science, 661, p.317 - 332, 2024/05
Times Cited Count:7 Percentile:79.28(Chemistry, Physical)no abstracts in English
 molecular dynamics simulations reveal the hydration structure of the radium(II) ion
molecular dynamics simulations reveal the hydration structure of the radium(II) ionYamaguchi, Akiko; Nagata, Kojiro*; Kobayashi, Keita; Tanaka, Kazuya; Kobayashi, Toru; Tanida, Hajime; Shimojo, Kojiro; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
iScience (Internet), 25(8), p.104763_1 - 104763_12, 2022/08
Times Cited Count:18 Percentile:68.88(Multidisciplinary Sciences)no abstracts in English
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
Yamaguchi, Akiko; Nagata, Kojiro*; Tanaka, Kazuya; Kobayashi, Keita; Kobayashi, Toru; Shimojo, Kojiro; Tanida, Hajime; Sekiguchi, Tetsuhiro; Kaneta, Yui; Matsuda, Shohei; et al.
Hosha Kagaku, (45), p.28 - 30, 2022/03
no abstracts in English
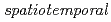 measurement and analysis techniques in X-ray photoelectron spectroscopy; From NAP-HARPES to 4D-XPS
measurement and analysis techniques in X-ray photoelectron spectroscopy; From NAP-HARPES to 4D-XPSToyoda, Satoshi*; Yamamoto, Tomoki*; Yoshimura, Masashi*; Sumida, Hirosuke*; Mineoi, Susumu*; Machida, Masatake*; Yoshigoe, Akitaka; Suzuki, Satoru*; Yokoyama, Kazushi*; Ohashi, Yuji*; et al.
Vacuum and Surface Science, 64(2), p.86 - 91, 2021/02
We have developed 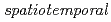 measurement and analysis techniques in X-ray photoelectron spectroscopy. To begin with, time-division depth profiles of gate stacked film interfaces have been achieved by NAP-HARPES (Near Ambient Pressure Hard X-ray Angle-Resolved Photo Emission Spectroscopy) data. We then have promoted our methods to quickly perform peak fittings and depth profiling from time-division ARPES data, which enables us to realize 4D-XPS analysis. It is found that the traditional maximum entropy method (MEM) combined with Jackknife averaging of sparse modeling in NAP-HARPES data is effective to perform dynamic measurement of depth profiles with high precision.
measurement and analysis techniques in X-ray photoelectron spectroscopy. To begin with, time-division depth profiles of gate stacked film interfaces have been achieved by NAP-HARPES (Near Ambient Pressure Hard X-ray Angle-Resolved Photo Emission Spectroscopy) data. We then have promoted our methods to quickly perform peak fittings and depth profiling from time-division ARPES data, which enables us to realize 4D-XPS analysis. It is found that the traditional maximum entropy method (MEM) combined with Jackknife averaging of sparse modeling in NAP-HARPES data is effective to perform dynamic measurement of depth profiles with high precision.
Minami, Ryutaro*; Kariya, Tsuyoshi*; Imai, Tsuyoshi*; Numakura, Tomoharu*; Endo, Yoichi*; Nakabayashi, Hidetaka*; Eguchi, Taku*; Shimozuma, Takashi*; Kubo, Shin*; Yoshimura, Yasuo*; et al.
Nuclear Fusion, 53(6), p.063003_1 - 063003_7, 2013/06
Minami, Ryutaro*; Kariya, Tsuyoshi*; Imai, Tsuyoshi*; Numakura, Tomoharu*; Endo, Yoichi*; Nakabayashi, Hidetaka*; Eguchi, Taku*; Shimozuma, Takashi*; Kubo, Shin*; Yoshimura, Yasuo*; et al.
Nuclear Fusion, 53(6), p.063003_1 - 063003_7, 2013/06
Times Cited Count:13 Percentile:47.20(Physics, Fluids & Plasmas) solutions; Model experiments for the chemical study of Db
solutions; Model experiments for the chemical study of DbKasamatsu, Yoshitaka; Toyoshima, Atsushi; Haba, Hiromitsu*; Tome, Hayato*; Tsukada, Kazuaki; Akiyama, Kazuhiko*; Yoshimura, Takashi*; Nagame, Yuichiro
Journal of Radioanalytical and Nuclear Chemistry, 279(2), p.371 - 376, 2009/02
Times Cited Count:13 Percentile:63.16(Chemistry, Analytical)Anion-exchange behavior of the group-5 elements Nb and Ta, and their pseudo homologue Pa in HF and HF/HNO solutions was investigated by a batch method to find suitable conditions for the anion-exchange experiment of element 105 (Dubnium, Db). We determined distribution coefficients of those elements on the anion-exchange resin as a function of the F
solutions was investigated by a batch method to find suitable conditions for the anion-exchange experiment of element 105 (Dubnium, Db). We determined distribution coefficients of those elements on the anion-exchange resin as a function of the F and NO
and NO
 concentrations. Clearly different anion-exchange behavior was observed among these elements. Based on the results, we discuss the fluoro-complex formation of each element and suggest experimental conditions for the study of fluoride complexation of Db.
concentrations. Clearly different anion-exchange behavior was observed among these elements. Based on the results, we discuss the fluoro-complex formation of each element and suggest experimental conditions for the study of fluoride complexation of Db.
Haba, Hiromitsu*; Akiyama, Takahiro*; Kaji, Daiya*; Kikunaga, Hidetoshi*; Kuribayashi, Takahiro*; Morimoto, Koji*; Morita, Kosuke*; Oe, Kazuhiro*; Sato, Nozomi*; Shinohara, Atsushi*; et al.
European Physical Journal D, 45(1), p.81 - 86, 2007/10
Times Cited Count:12 Percentile:51.13(Optics)A review is given on the startup of the superheavy element (SHE) chemistry at RIKEN. A gas-jet transport system for the SHE chemistry has been coupled to the gas-filled recoil ion separator GARIS at the RIKEN Linear Accelerator. The performance of the system was appraised using  Fr and
Fr and 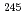 Fm produced in the
Fm produced in the 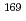 Tm(
Tm(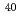 Ar,3
Ar,3 )
) Fr and
Fr and  Pb(
Pb(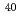 Ar,3
Ar,3 )
)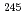 Fm reactions, respectively. The
Fm reactions, respectively. The  particles of
particles of  Fr and
Fr and 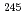 Fm separated with GARIS and transported by the gas-jet were identified with a rotating wheel system for
Fm separated with GARIS and transported by the gas-jet were identified with a rotating wheel system for  spectrometry under desired low background condition. The high gas-jet efficiencies over 80% were independent of the beam intensities up to 2 particle
spectrometry under desired low background condition. The high gas-jet efficiencies over 80% were independent of the beam intensities up to 2 particle  A. A gas-jet coupled target system for the production of SHEs was also installed on the beam line of the RIKEN K70 AVF cyclotron. The gas-jet transport of
A. A gas-jet coupled target system for the production of SHEs was also installed on the beam line of the RIKEN K70 AVF cyclotron. The gas-jet transport of  No and
No and  Rf produced in the
Rf produced in the  U(
U( Ne,5
Ne,5 )
) No and
No and  Cm(
Cm( O,5
O,5 )
) Rf reactions, respectively, was conducted for the future chemical studies of
Rf reactions, respectively, was conducted for the future chemical studies of  Sg via the
Sg via the  Cm(
Cm( Ne, 5
Ne, 5 )
) Sg reaction.
Sg reaction.
Yamada, Hidenori*; Tamada, Taro; Kosaka, Megumi*; Miyata, Kohei*; Fujiki, Shinya*; Tano, Masaru*; Moriya, Masayuki*; Yamanishi, Mamoru*; Honjo, Eijiro; Tada, Horiko*; et al.
Protein Science, 16(7), p.1389 - 1397, 2007/07
Times Cited Count:41 Percentile:60.11(Biochemistry & Molecular Biology)In an attempt to control protein incorporation in a crystal lattice, a leucine zipper-like hydrophobic interface (comprising four leucine residues) was introduced into a helical region (helix 2) of the human pancreatic ribonuclease 1 (RNase 1) that was predicted to form a suitable crystallization interface. Although crystallization of wild type RNase 1 has not yet been reported, the RNase 1 mutant having four leucines (4L-RNase 1) was successfully crystallized under several different conditions. The crystal structures were subsequently determined by X-ray crystallography by molecular replacement using the structure of bovine RNase A. The overall structure of 4L-RNase 1 is quite similar to that of the bovine RNase A, and the introduced leucine residues formed the designed crystal interface. To further characterize the role of the introduced leucine residues in crystallization of RNase 1, the number of leucines was reduced to three or two (3L- and 2L-RNase 1, respectively). Both mutants crystallized and a similar hydrophobic interface as in 4L-RNase 1 was observed. A related approach to engineer crystal contacts at helix 3 of RNase 1 (N4L-RNase 1) was also evaluated. N4L-RNase 1 also successfully crystallized, and formed the expected hydrophobic packing interface. These results suggest that appropriate introduction of a leucine zipper-like hydrophobic interface can promote intra molecular symmetry for more efficient protein crystallization in crystal lattice engineering efforts.
Isobe, Mitsutaka*; Toi, Kazuo*; Matsushita, Hiroyuki*; Goto, Kazuyuki*; Suzuki, Chihiro*; Nagaoka, Kenichi*; Nakajima, Noriyoshi*; Yamamoto, Satoshi*; Murakami, Sadayoshi*; Shimizu, Akihiro*; et al.
Nuclear Fusion, 46(10), p.S918 - S925, 2006/10
Times Cited Count:31 Percentile:68.97(Physics, Fluids & Plasmas)no abstracts in English
 (
(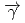 ,
,
 )
)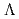 and
and  (
(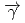 ,
,
 )
)
 reactions for
reactions for 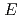
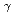 =1.5-2.4 GeV
=1.5-2.4 GeVZegers, R. G. T.*; Sumihama, Mizuki*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Dat , S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
Physical Review Letters, 91(9), p.092001_1 - 092001_4, 2003/08
Times Cited Count:128 Percentile:94.59(Physics, Multidisciplinary)no abstracts in English
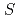 = +1 Baryon resonance in photoproduction from the neutron
= +1 Baryon resonance in photoproduction from the neutronNakano, Takashi*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Date, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; Fujiwara, Mamoru; et al.
Physical Review Letters, 91(1), p.012002_1 - 012002_4, 2003/07
Times Cited Count:1023 Percentile:99.84(Physics, Multidisciplinary)no abstracts in English
Takeda, Toshikazu*; Ito, Noboru*; Kugo, Teruhiko*; Takamoto, Masanori*; Aoki, Shigeaki*; Kawagoe, Yoshihiro*; Sengoku, Katsuhisa*; Tanaka, Motonari*; Yoshimura, Akira*; Tamitani, Masashi*; et al.
PNC TJ2605 89-001, 251 Pages, 1989/03
no abstracts in English
Toyoshima, Atsushi; Kanda, Akimitsu*; Ikeda, Takumi*; Yoshimura, Takashi*; Shinohara, Atsushi*; Yano, Shinya*; Komori, Yukiko*; Haba, Hiromitsu*
no journal, ,
Astatine (At) is reported to be bound in a few oxidation states in aqueous solutions. However, their valencies and chemical species are experimentally not identified. In this study, we studied redox behavior of At using redox agents and using a flow electrolytic column.  At was produced in the
At was produced in the 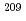 Bi(
Bi( , 2
, 2 )
) At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO
At reaction and was then separated from the target by a distillation method. Then, redox of At was carried out in 1.0 M HClO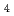 using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.
using redox agents or by an electrolysis. After the redox, solvent extraction with HDEHP was performed to identify the redox reactions. Using the redox agents, extraction yields of At were different between the conditions with oxidizing or reducing agents and without redox agents. This means that redox reactions of At can be identified under the present extraction conditions. On the other hand, by the electrolysis, extraction yields of At were almost constant against the variation of applied potential. This is possibly due to the restoration to the original oxidation state after the electrolysis. In the future, we will perform flow electrolytic column chromatography available for simultaneous electrolysis and separation.
Yokobori, Shinichi*; Yang, Y.*; Sugino, Tomohiro*; Kawaguchi, Yuko*; Itahashi, Shiho*; Fujisaki, Kenta*; Fushimi, Hidehiko*; Hasegawa, Sunao*; Hashimoto, Hirofumi*; Hayashi, Nobuhiro*; et al.
no journal, ,
Yokokita, Takuya*; Oe, Kazuhiro; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Nakamura, Kohei*; Kasamatsu, Yoshitaka*; Takahashi, Naruto*; Yoshimura, Takashi*; Takamiya, Koichi*; et al.
no journal, ,
We carried out solvent extraction of Mo(VI), Mo(V), W(VI) and W(V) in 0.01-0.36 M Aliquat 336 / 0.1-11 M HCl system as model experiments for element 106, seaborgium (Sg). The HCl solutions of Mo(VI), Mo(V), W(VI) and W(V) were prepared using macro amounts of Mo and W. Extraction behaviors of mononuclear Mo and W were also investigated using carrier-free radiotracers  Mo and
Mo and  W, which were produced as
W, which were produced as  U(n, f) and
U(n, f) and  Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Ta (p, n) reaction, respectively. The distribution ratios (D) of Mo(V) and W(V) were higher than those of Mo(VI) and W(VI), respectively. These results suggest that reduction behavior of the group-6 elements can be observed by solvent extraction. The D values of carrier-free Mo(VI) and W(VI) are almost the same as those with macro amounts in 6-11 M HCl. This condition would be suitable for the Sg experiments.
Ko, B.-S.*; Hasegawa, Shin; Hamada, Takashi; Yoshimura, Kimio; Maekawa, Yasunari
no journal, ,
Yokokita, Takuya*; Komori, Yukiko*; Kikutani, Yuki*; Kino, Aiko*; Shiohara, Naoya*; Kasamatsu, Yoshitaka*; Yoshimura, Takashi*; Takahashi, Naruto*; Oe, Kazuhiro; Shinohara, Atsushi*
no journal, ,
We are planning to study a redox behavior of element 106, seaborgium (Sg) based on the comparison of the behaviors with those of molybdenum (Mo) and tungsten (W). In this work, we studied solvent extraction behaviors of Mo(VI), Mo(V), W(VI), and W(V) with Aliquat 336 from hydrochloric acid (HCl). Mo(V) is extracted with higher distribution ratios than those of Mo(VI) in 4 to 11 M HCl. Distribution ratios of Mo(V) obtained by reducing Mo(VI) electrochemically are in good agreement with those of [MoCl ].
].
Takeuchi, Kota*; Hamada, Takashi; Yoshimura, Kimio; Hiroki, Akihiro; Maekawa, Yasunari
no journal, ,
no abstracts in English