Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sekine, Yurina; Nankawa, Takuya; Hiroi, Kosuke; Oba, Yojiro*; Nagakawa, Yoshiyasu*; Sugita, Tsuyoshi; Shibayama, Yuki; Ikeda-Fukazawa, Tomoko*
Carbohydrate Polymers, 327, p.121538_1 - 121538_11, 2024/03
Times Cited Count:0 Percentile:0.01(Chemistry, Applied)We describe non-toxic, tough nanocellulose (NC) hydrogels formed from chemically unmodified NC by cellulose crystalline transformation and subsequent freeze cross-linking reaction. Using low-concentration NaOH and freezing together induced the crystalline transformation of NC from cellulose I to II via freeze concentration. After the crystalline transformation, cross-linking between the NC and CA in the freeze concentration layer (FCL) provided a strong NC network structure, forming NC hydrogels with high mechanical strength. The freeze-cross-linked NC hydrogel easily retained powder adsorbents in its inner space by mixing the NC-NaOH sol and the powder, and the hydrogel showed high removal efficiency for heavy metals. The results highlight the versatility of chemically unmodified celluloses in developing functional materials, suggest possible practical applications.
 Mo/
Mo/ Tc generators using the (n,
Tc generators using the (n, ) method (Thesis)
) method (Thesis)Fujita, Yoshitaka
JAEA-Review 2023-010, 108 Pages, 2023/08
 Tc (technetium-99m) is the most widely used radioisotope in radiopharmaceutical and is decayed from the parent nuclide
Tc (technetium-99m) is the most widely used radioisotope in radiopharmaceutical and is decayed from the parent nuclide  Mo (molybdenum-99). Most of
Mo (molybdenum-99). Most of  Mo is generated as one of the fission products of uranium, but recently, from the viewpoint of nuclear security and nuclear nonproliferation, a uranium-free
Mo is generated as one of the fission products of uranium, but recently, from the viewpoint of nuclear security and nuclear nonproliferation, a uranium-free  Mo production method is desired. One such method is the (n,
Mo production method is desired. One such method is the (n, ) method, in which
) method, in which 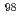 Mo is irradiated by neutrons. However, since the specific activity of
Mo is irradiated by neutrons. However, since the specific activity of  Mo produced by this method is extremely low, it is necessary to improve the Mo adsorption and
Mo produced by this method is extremely low, it is necessary to improve the Mo adsorption and  Tc elution property of alumina (Al
Tc elution property of alumina (Al O
O ), which is used as a Mo adsorbent, to apply this method to the
), which is used as a Mo adsorbent, to apply this method to the  Mo/
Mo/ Tc generator, a device for separation and concentration of
Tc generator, a device for separation and concentration of  Tc from
Tc from  Mo. Therefore, the objective of this thesis is to elucidate the parameters effective for improving the performance of alumina for the practical use of the
Mo. Therefore, the objective of this thesis is to elucidate the parameters effective for improving the performance of alumina for the practical use of the  Mo/
Mo/ Tc generator using the (n,
Tc generator using the (n, ) method, and to contribute to the development of alumina columns that may be applicable to low specific activity
) method, and to contribute to the development of alumina columns that may be applicable to low specific activity  Mo. In this study, alumina with different starting materials was prepared and its applicability as Mo adsorbent for
Mo. In this study, alumina with different starting materials was prepared and its applicability as Mo adsorbent for  Mo/
Mo/ Tc generator was evaluated. The effects of crystal structure and specific surface area of alumina on Mo adsorption properties were clarified, and the Mo adsorption mechanism was elucidated based on the results of surface analysis of alumina. In addition,
Tc generator was evaluated. The effects of crystal structure and specific surface area of alumina on Mo adsorption properties were clarified, and the Mo adsorption mechanism was elucidated based on the results of surface analysis of alumina. In addition,  Tc elution properties and
Tc elution properties and  Tc solution quality were evaluated using MoO
Tc solution quality were evaluated using MoO irradiated in the Kyoto University Research Reactor (KUR), and a new column shape with potential application to generators was proposed based on the experiment results of alumina columns designed for current generators.
irradiated in the Kyoto University Research Reactor (KUR), and a new column shape with potential application to generators was proposed based on the experiment results of alumina columns designed for current generators.
Zheng, X.*; Kato, Masaru*; Uemura, Yohei*; Matsumura, Daiju; Yagi, Ichizo*; Takahashi, Kiyonori*; Noro, Shinichiro*; Nakamura, Takayoshi*
Inorganic Chemistry, 62(3), p.1257 - 1263, 2023/01
Times Cited Count:1 Percentile:47.62(Chemistry, Inorganic & Nuclear) Mo/
Mo/ Tc generator by (n,
Tc generator by (n,  ) method, 4
) method, 4Fujita, Yoshitaka; Seki, Misaki; Ngo, M. C.*; Do, T. M. D.*; Hu, X.*; Yang, Y.*; Takeuchi, Tomoaki; Nakano, Hiroko; Fujihara, Yasuyuki*; Yoshinaga, Hisao*; et al.
KURNS Progress Report 2021, P. 118, 2022/07
no abstracts in English
Nankawa, Takuya; Sekine, Yurina; Yamada, Teppei*
Bulletin of the Chemical Society of Japan, 95(5), p.825 - 829, 2022/05
Times Cited Count:2 Percentile:26.09(Chemistry, Multidisciplinary)Advances in hazardous metal ion removal are essential for wastewater clean-up to tackle the global water shortage crisis. Here, we report a Pb-selective adsorbent using a Tb oxalate framework (TOF) synthesized by a one-pot hydrothermal method. The TOF has a two-dimensional sheet structure, in which the interlayer space functions as an ion exchangeable site. Sorption tests using a mixed-ion solution containing Pb , Cd
, Cd , Mn
, Mn , Co
, Co , Ni
, Ni , Cu
, Cu , Na
, Na , K
, K , Mg
, Mg , and Ca
, and Ca showed that the TOF has high selectivity for Pb
showed that the TOF has high selectivity for Pb among other metal ions. The saturated adsorption capacity of the TOF for Pb
among other metal ions. The saturated adsorption capacity of the TOF for Pb was 276 mg g
was 276 mg g , which is higher than that of conventional adsorbents. Furthermore, the TOF exhibited reversible Pb
, which is higher than that of conventional adsorbents. Furthermore, the TOF exhibited reversible Pb adsorption/desorption and could be used for at least three cycles. The results showed that TOF has excellent potential as an adsorbent for removing Pb
adsorption/desorption and could be used for at least three cycles. The results showed that TOF has excellent potential as an adsorbent for removing Pb , and because of its reusability, it is also a promising material for wastewater clean-up.
, and because of its reusability, it is also a promising material for wastewater clean-up.
Kumagai, Yuta; Kimura, Atsushi*; Taguchi, Mitsumasa*; Watanabe, Masayuki
Radiation Physics and Chemistry, 191, p.109831_1 - 109831_8, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)In this study, we investigated and compared the effects of a high-silica zeolite (HMOR) on the radiation-induced degradation of three aromatic chlorides, 2-chlorophenol (2-ClPh), 2-chloroaniline (2-ClAn), and 2-chlorobenzoic acid (2-ClBA), in order to examine its potential to reduce the influence of ions in water matrix in the irradiation treatment of water-soluble organic compounds. In the presence of ions reactive to radicals, the degradation of 2-ClPh in water was inhibited, but the combined use of HMOR much improved the degradation yield. This improvement was attributed to high performance of HMOR in adsorption of 2-ClPh. Similarly, HMOR was effective for adsorption of 2-ClAn and facilitated the 2-ClAn degradation by irradiation. In contrast, HMOR was poor at adsorption of 2-ClBA and consistently the degradation of 2-ClBA in the water-HMOR mixture was inhibited by the radical scavenger. These results demonstrate that HMOR can mitigate the influence of radical scavengers in water.
Fujita, Yoshitaka; Niizeki, Tomotake*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; Yamauchi, Yusuke*; Malgras, V.*; Kaneti, Y. V.*; Liu, C.-H.*; Hatano, Kentaro*; Suematsu, Hisayuki*; et al.
Bulletin of the Chemical Society of Japan, 95(1), p.129 - 137, 2022/01
Times Cited Count:8 Percentile:72.56(Chemistry, Multidisciplinary)In this work, the mechanisms responsible for the adsorption of molybdate ions on alumina are investigated using in-depth surface analyses carried out on alumina specimens immersed in solutions containing different molybdate ions at different pH values. The obtained results reveal that when alumina is immersed in an acidic solution containing molybdate ions, the hydroxyl groups present on the surface are removed to generate positively charged sites, and molybdate ions (MoO
 or AlMo
or AlMo O
O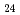 H
H
 ) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo
) are adsorbed by electrostatic interaction. Alumina dissolves slightly in an acidic solution to form AlMo O
O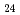 H
H
 , which is more easily desorbed than MoO
, which is more easily desorbed than MoO
 . Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
. Furthermore, the enhancement in the Mo adsorption or desorption property may be achieved by enriching the surface of the alumina adsorbent with many -OH groups and optimizing Mo solution to adsorb molybdate ions on alumina as MoO
 ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope (
ions. These findings will assist researchers in engineering more efficient and stable alumina-based adsorbents for molybdenum adsorption used in medical radioisotope ( Mo/
Mo/ Tc) generators.
Tc) generators.
 Mo adsorption and
Mo adsorption and  Tc elution with alumina columns
Tc elution with alumina columnsFujita, Yoshitaka; Seki, Misaki; Sano, Tadafumi*; Fujihara, Yasuyuki*; Suzuki, Tatsuya*; Yoshinaga, Hisao*; Hori, Junichi*; Suematsu, Hisayuki*; Tsuchiya, Kunihiko
Journal of Physics; Conference Series, 2155, p.012018_1 - 012018_6, 2022/01
Technetium-99m ( Tc), the daughter nuclide of Molybdenum-99 (
Tc), the daughter nuclide of Molybdenum-99 ( Mo), is the most commonly used radioisotope in radiopharmaceuticals. The research and development (R&D) for the production of
Mo), is the most commonly used radioisotope in radiopharmaceuticals. The research and development (R&D) for the production of  Mo by the neutron activation method ((n,
Mo by the neutron activation method ((n,  ) method) has been carried out from viewpoints of no-proliferation and nuclear security, etc. Since the specific activity of
) method) has been carried out from viewpoints of no-proliferation and nuclear security, etc. Since the specific activity of  Mo produced by the (n,
Mo produced by the (n,  ) method is extremely low, developing Al
) method is extremely low, developing Al O
O with a large Mo adsorption capacity is necessary to adapt (n,
with a large Mo adsorption capacity is necessary to adapt (n,  )
) Mo to the generator. In this study, three kinds of Al
Mo to the generator. In this study, three kinds of Al O
O specimens with different raw materials were prepared and compared their adaptability to generators by static and dynamic adsorption. MoO
specimens with different raw materials were prepared and compared their adaptability to generators by static and dynamic adsorption. MoO pellet pieces (1.5g) were irradiated with 5 MW for 20 min in the Kyoto University Research Reactor (KUR). Irradiated MoO
pellet pieces (1.5g) were irradiated with 5 MW for 20 min in the Kyoto University Research Reactor (KUR). Irradiated MoO pellet pieces were dissolved in 6M-NaOH aq. In dynamic adsorption, 1 g of Al
pellet pieces were dissolved in 6M-NaOH aq. In dynamic adsorption, 1 g of Al O
O was filled into a PFA tube (
was filled into a PFA tube (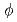 1.59 mm). The
1.59 mm). The  Mo adsorption capacity of Al
Mo adsorption capacity of Al O
O specimens under dynamic condition was slightly reduced compared to that under static condition. The
specimens under dynamic condition was slightly reduced compared to that under static condition. The  Tc elution rate was about 100% at 1.5 mL of milking in dynamic adsorption, while it was around 56-87% in static adsorption. The
Tc elution rate was about 100% at 1.5 mL of milking in dynamic adsorption, while it was around 56-87% in static adsorption. The  Mo/
Mo/ Tc ratio of dynamic condition was greatly reduced compared to that of static condition. Therefore, the
Tc ratio of dynamic condition was greatly reduced compared to that of static condition. Therefore, the  Tc elution property is greatly affected by the method of adsorbing Mo, e.g., the column shape, the linear flow rate, etc.
Tc elution property is greatly affected by the method of adsorbing Mo, e.g., the column shape, the linear flow rate, etc.
Nankawa, Takuya; Sekine, Yurina
Isotope News, (778), p.34 - 35, 2021/12
A high-performance adsorbent was synthesized by reaction of waste bone and sodium hydrogen carbonate. Since waste bones are inexpensive and well-supplied materials, it has the potential to be used for a wide range of decontamination and removal of harmful metals.
Hayashi, Natsuki*; Matsumura, Daiju; Hoshina, Hiroyuki*; Ueki, Yuji*; Tsuji, Takuya; Chen, J.*; Seko, Noriaki*
Separation and Purification Technology, 277, p.119536_1 - 119536_8, 2021/12
Times Cited Count:15 Percentile:65.52(Engineering, Chemical)Project 6 Meeting Members for Tsukuba International Strategic Zone
JAEA-Review 2021-016, 102 Pages, 2021/11
In December 2011, the Prime Minister designated Tsukuba and some areas in Ibaraki Prefecture as "Comprehensive Special Zones". In the Tsukuba International Strategic Zone, nine advanced research and development (R&D) projects are underway with the goal of promoting industrialization of life innovation and green innovation utilizing the science and technology in Tsukuba. In these projects, the domestic production of medical radioisotope (Technetium-99m,  Tc) was certified as a new project in October 2013, and R&D have been performed in collaboration with related organizations with Japan Atomic Energy Agency (JAEA) as the project leader. Japan is the third largest consumer of molybdenum-99 (
Tc) was certified as a new project in October 2013, and R&D have been performed in collaboration with related organizations with Japan Atomic Energy Agency (JAEA) as the project leader. Japan is the third largest consumer of molybdenum-99 ( Mo) after the United States and Europe, and all
Mo) after the United States and Europe, and all  Mo are imported. Supply will be insufficient if overseas reactors are shut down due to trouble or if transportation (air and land transportations) is stopped due to volcanic eruptions and some accidents. Thus, early domestic production of
Mo are imported. Supply will be insufficient if overseas reactors are shut down due to trouble or if transportation (air and land transportations) is stopped due to volcanic eruptions and some accidents. Thus, early domestic production of  Mo is strongly required. This project is a technology development aimed at domestic production of
Mo is strongly required. This project is a technology development aimed at domestic production of  Mo, which is a raw material of
Mo, which is a raw material of  Tc used as a diagnostic agent. This report summarizes the activities carried out in the first and second phase of the domestic production of medical radioisotope (
Tc used as a diagnostic agent. This report summarizes the activities carried out in the first and second phase of the domestic production of medical radioisotope ( Tc) (here referred to as the "Project 6") in Tsukuba International Strategic Zone (FY2014-2020).
Tc) (here referred to as the "Project 6") in Tsukuba International Strategic Zone (FY2014-2020).
Okamoto, Yoshihiro; Osugi, Takeshi; Shiwaku, Hideaki; Akabori, Mitsuo*
Insights Concerning the Fukushima Daiichi Nuclear Accident, Vol.4; Endeavors by Scientists, p.285 - 294, 2021/10
The transfer behavior of cesium adsorbed on some clay minerals in aqueous solution was investigated by X-ray absorption fine structure (XAFS) analysis of the Cs K-edge. The sample was prepared by mixing Cs-adsorbed mineral with a different pure clay mineral in water. The XAFS results of the dried mixture powder were compared with those obtained before mixing. It was recognized from the XAFS analysis for three kinds of clay minerals illite, kaolinite and vermiculite, that cesium was transferred from kaolinite to illite and vermiculite, and from illite to vermiculite. It can be concluded that cesium is transferred to and accumulated in vermiculite.
 -P for an advanced reprocessing
-P for an advanced reprocessingHoriuchi, Yusuke; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Kida, Fukuka*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 330(1), p.237 - 244, 2021/10
Times Cited Count:7 Percentile:75.12(Chemistry, Analytical)Applicability of tetra2-ehylhexyl diglycolamide (TEHDGA) impregnated adsorbent for minor actinide (MA) recovery from high level liquid waste (HLLW) in extraction chromatography technology was investigated through batch-wise adsorption and column separation experiments. Distribution ratio of representative fission product elements were obtained by the batch-wise experiments, and TEHDGA adsorbent was shown to be preferable to TODGA adsorbent for decontamination of several species. All Ln(III) supplied into the TEHDGA adsorbent packed column was properly eluted from the column, and the applicability of the adsorbent was successfully showed by this study.
Sekine, Yurina; Nankawa, Takuya
Chem-Station (Internet), 1 Pages, 2021/03
We will explain the experimental results and the background of the research that developed a highly efficient toxic metal adsorbent using wastebone as a raw material.
 Mo-adsorption/
Mo-adsorption/ Tc-elution properties of alumina with different surface structures
Tc-elution properties of alumina with different surface structuresFujita, Yoshitaka; Seki, Misaki; Sano, Tadafumi*; Fujihara, Yasuyuki*; Kitagawa, Tomoya*; Matsukura, Minoru*; Hori, Junichi*; Suzuki, Tatsuya*; Tsuchiya, Kunihiko
Journal of Radioanalytical and Nuclear Chemistry, 327(3), p.1355 - 1363, 2021/03
Times Cited Count:3 Percentile:43.41(Chemistry, Analytical)We prepared three types of Al O
O with different surface structures and investigated
with different surface structures and investigated  Mo-adsorption/
Mo-adsorption/ Tc-elution properties using [
Tc-elution properties using [ Mo]MoO
Mo]MoO that was irradiated in the Kyoto University Research Reactor. Al
that was irradiated in the Kyoto University Research Reactor. Al O
O adsorbed [
adsorbed [ Mo]molybdate ions in solutions at different pH; the lower was the pH, the higher was the Mo-adsorption capacity of Al
Mo]molybdate ions in solutions at different pH; the lower was the pH, the higher was the Mo-adsorption capacity of Al O
O . The
. The  Tc-elution properties of molybdate ion adsorbed Al
Tc-elution properties of molybdate ion adsorbed Al O
O were elucidated by flowing saline. Consequently, it was suggested that
were elucidated by flowing saline. Consequently, it was suggested that  Mo-adsorption/desorption properties are affected by the specific surface of Al
Mo-adsorption/desorption properties are affected by the specific surface of Al O
O and
and  Tc-elution properties are affected by the crystal structure of Al
Tc-elution properties are affected by the crystal structure of Al O
O .
.
Benu, D. P.*; Earnshaw, J.*; Ashok, A.*; Tsuchiya, Kunihiko; Saptiama, I.*; Yuliarto, B.*; Suendo, V.*; Mukti, R. R.*; Fukumitsu, Nobuyoshi*; Ariga, Katsuhiko*; et al.
Bulletin of the Chemical Society of Japan, 94(2), p.502 - 507, 2021/02
Times Cited Count:11 Percentile:64.63(Chemistry, Multidisciplinary)no abstracts in English
Takai, Shizuka; Kimura, Hideo*; Uchikoshi, Emiko*; Munakata, Masahiro; Takeda, Seiji
JAEA-Data/Code 2020-007, 174 Pages, 2020/09
The MIG2DF computer code is a computer program that simulates groundwater flow and radionuclide transport in porous media for the safety assessment of radioactive waste disposal. The original version of MIG2DF was released in 1992. The original code employs a two-dimensional (vertical or horizontal cross-section, or an axisymmetric configuration) finite-element method to approximate the governing equations for density-dependent saturated-unsaturated groundwater flow and radionuclide transport. Meanwhile, for geological disposal of radioactive wastes, landscape evolution such as uplift and erosion needs to be assessed as a long-term geological and climate events, considering site conditions. In coastal areas, the impact to groundwater flow by change of salinity distribution to sea level change also needs to be considered. To deal with these events in the assessment, we have revised the original version of MIG2DF and developed the external program which enables MIG2DF to consider unsteady landscape evolution. In these developments, this report describes an upgrade of MIG2DF (Version 2) and presents the configuration, equations, methods, and verification. This reports also give the explanation external programs of MIG2DF: PASS-TRAC (the particle tracking code), PASS-PRE (the code for dataset preparation), and PASS-POST (the post-processing visualization system).
 Mo/
Mo/ Tc generator by (n,
Tc generator by (n, ) method, 2
) method, 2Fujita, Yoshitaka; Seki, Misaki; Namekawa, Yoji*; Nishikata, Kaori; Kato, Yoshiaki; Sayato, Natsuki; Tsuchiya, Kunihiko; Sano, Tadafumi*; Fujihara, Yasuyuki*; Hori, Junichi*; et al.
KURNS Progress Report 2019, P. 157, 2020/08
no abstracts in English
Rizaal, M.; Saito, Takumi*; Okamoto, Koji*; Erkan, N.*; Nakajima, Kunihisa; Osaka, Masahiko
Mechanical Engineering Journal (Internet), 7(3), p.19-00563_1 - 19-00563_10, 2020/06
The adsorption of cesium (Cs) on calcium silicate insulation of primary piping system is postulated to contribute in high dose rate of surrounding pedestal area in Fukushima Daiichi NPP unit 2. In this study, room-temperature experiment of Cs adsorption on calcium silicate has been studied as an initial approach of Cs adsorption behavior toward higher temperature condition. As the result of analyzing of Cs adsorption kinetics, it was expected that the underlying adsorption mechanism is chemisorption. Furthermore, analysis of adsorption isotherm suggested unrestricted monolayer formation followed by multilayer formation.
 I impacted by atmospheric
I impacted by atmospheric  I releases from a spent nuclear fuel reprocessing plant
I releases from a spent nuclear fuel reprocessing plantOta, Masakazu; Terada, Hiroaki; Hasegawa, Hidenao*; Kakiuchi, Hideki*
Science of the Total Environment, 704, p.135319_1 - 135319_15, 2020/02
Times Cited Count:6 Percentile:28.61(Environmental Sciences)Land-surface transfers of  I are modeled and incorporated into a land-surface model (SOLVEG-II), and the model was applied to the observed transfer of
I are modeled and incorporated into a land-surface model (SOLVEG-II), and the model was applied to the observed transfer of  I at a vegetated field impacted by atmospheric releases of
I at a vegetated field impacted by atmospheric releases of  I from Rokkasho reprocessing plant during 2007 to investigate the importance of each
I from Rokkasho reprocessing plant during 2007 to investigate the importance of each  I-transfer pathway. The model calculation revealed that contamination of leaves of wild bamboo grasses was mostly caused by foliar adsorption of
I-transfer pathway. The model calculation revealed that contamination of leaves of wild bamboo grasses was mostly caused by foliar adsorption of  I (81%) induced via wet deposition of
I (81%) induced via wet deposition of  I. Wet deposition of
I. Wet deposition of  I was the main
I was the main  I-input to the soil, ten-fold the dry deposition of
I-input to the soil, ten-fold the dry deposition of  I
I ; however, the deposition of
; however, the deposition of  I during 2007 was only 2% of the model-assumed
I during 2007 was only 2% of the model-assumed  I that pre-existed in the soil; indicating the importance of long-term accumulation of
I that pre-existed in the soil; indicating the importance of long-term accumulation of  I in soils. The model calculation also revealed that root uptake of
I in soils. The model calculation also revealed that root uptake of  I, not methylation, control the long-term turnover of soil
I, not methylation, control the long-term turnover of soil  I.
I.