Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 mesocrystal formation
mesocrystal formationLi, Z.*; Piankova, D.*; Yang, Y.*; Kumagai, Yuta; Zschiesche, H.*; Jonsson, M.*; Tarakina, N. V.*; Soroka, I. L.*
Angewandte Chemie; International Edition, 61(6), p.e202112204_1 - e202112204_9, 2022/02
Times Cited Count:7 Percentile:35.84(Chemistry, Multidisciplinary)The role of intermediate phases in CeO mesocrystal formation from aqueous Ce(III) solutions subjected to
mesocrystal formation from aqueous Ce(III) solutions subjected to  -radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO
-radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO
mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
Zhang, W. Q.*; Yamaguchi, Toshio*; Fang, C. H.*; Yoshida, Koji*; Zhou, Y. Q.*; Zhu, F. Y.*; Machida, Shinichi*; Hattori, Takanori; Li, W.*
Journal of Molecular Liquids, 348, p.118080_1 - 118080_11, 2022/02
Times Cited Count:3 Percentile:15.57(Chemistry, Physical)The ion hydration and association and hydrogen-bonded water structure in an aqueous 3 mol/kg RbCl solution were investigated at 298 K/0.1 MPa, 298 K/1 GPa, 523 K/1 GPa, and 523 K/4 GPa by neutron diffraction combined with EPSR methods. The second hydration layer of Rb and Cl
and Cl becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb
becomes evident under elevated pressure and temperature conditions. The average oxygen coordination number of Rb (Cl
(Cl ) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb
) in the first hydration layer increases from 6.3 (5.9) ambient pressure to 8.9 (9.1) at 4 GPa, while decreasing coordination distance from 0.290 nm (0.322 nm) to 0.288 nm (0.314 nm). The orientation of the water dipole in the first solvation shell of Rb and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl
and a central water molecule is sensitive to pressure, but that in the first solvation shell of Cl does not change very much. The number of contact-ion pairs Rb
does not change very much. The number of contact-ion pairs Rb -Cl
-Cl decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
decreases with elevated temperature and increases with elevated pressure. Water molecules are closely packed, and the tetrahedral hydrogen-bonded network of water molecules no longer exists in extreme conditions.
Tominaga, Taiki*; Sahara, Masae*; Kawakita, Yukinobu; Nakagawa, Hiroshi; Yamada, Takeshi*
Journal of Applied Crystallography, 54(6), p.1631 - 1640, 2021/12
Times Cited Count:6 Percentile:58.26(Chemistry, Multidisciplinary)Segawa, Tomoomi; Kawaguchi, Koichi; Ishii, Katsunori; Suzuki, Masahiro; Fukasawa, Tomonori*; Fukui, Kunihiro*
Funtai Kogakkai-Shi, 57(9), p.485 - 494, 2020/09
In the spent fuel reprocessing process, a mixed solution of uranyl nitrate and plutonium nitrate is converted into mixed oxide powder by the microwave heating. To evaluate the applicability to the industrial-scale and acquire the characteristics data of the microwave heating denitration of various metal nitrate aqueous solutions based on the knowledge studied in the development of laboratory-scale basic experiments, the microwave heating characteristics and metal oxide powder properties were investigated using cerium nitrate, cobalt nitrate and copper nitrate aqueous solutions. The progress rate of the denitration reaction was depended on the position, and the denitration reaction proceeded faster at the periphery than at the center. The morphologies of the synthesized products were porous and hard dry solid with cerium nitrate aqueous solution, foamed dry solid with cobalt nitrate aqueous solution, and powdery particles with copper nitrate aqueous solution. The denitration ratio and average particle size of the synthesized products increased in the order of the cerium nitrate aqueous solution, the cobalt nitrate aqueous solution, and the copper nitrate aqueous solution. The numerical simulations revealed that the periphery of the bottom surface of the metal nitrate aqueous solution was heated by microwaves. This results consistent with the experimental results in which the denitration reaction started from the periphery of the metal nitrate aqueous solution.
 -radiation and H
-radiation and H O
O induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acid
induced oxidative dissolution of uranium(IV) oxide in aqueous solution containing phthalic acidKumagai, Yuta; Jonsson, M.*
Dalton Transactions (Internet), 49(6), p.1907 - 1914, 2020/02
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)This study aims to reveal possible involvements of organic acids in the oxidative dissolution of UO . Using phthalic acid as a model compound, we have measured adsorption on UO
. Using phthalic acid as a model compound, we have measured adsorption on UO and investigated effects on the reaction between H
and investigated effects on the reaction between H O
O and UO
and UO and on oxidative dissolution induced by
and on oxidative dissolution induced by  -irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H
-irradiation. Significant adsorption of phthalic acid was observed even at neutral pH. However, the reaction between H O
O and UO
and UO in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO
in phthalic acid solution induced oxidative dissolution of U(VI) similarly to aqueous bicarbonate solution. These results indicate that even though phthalic acid adsorbs on the UO surface, it is not involved in the interfacial reaction by H
surface, it is not involved in the interfacial reaction by H O
O . In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H
. In contrast, the dissolution of U by irradiation was inhibited in aqueous phthalic acid solution, whereas H O
O generated by radiolysis was consumed by UO
generated by radiolysis was consumed by UO . The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO
. The inhibition suggests that radical species derived from phthalic acid was involved in the redox reaction process of UO .
.
Yamaguchi, Toshio*; Nishino, Masaaki*; Yoshida, Koji*; Takumi, Masaharu*; Nagata, Kiyofumi*; Hattori, Takanori
European Journal of Inorganic Chemistry, 2019(8), p.1170 - 1177, 2019/02
Times Cited Count:18 Percentile:67.90(Chemistry, Inorganic & Nuclear)Neutron diffraction measurements of an aqueous 2 mol dm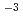 CaCl
CaCl solutions in D
solutions in D O have been made at 1 GPa, 298 K as well as 0.1 MPa, 298 K. The experimental structure factors are subjected to Empirical Potential Structure Refinement (EPSR) modeling to reveal the ion hydration and association and solvent water at the atomic level. About seven water molecules surround Ca
O have been made at 1 GPa, 298 K as well as 0.1 MPa, 298 K. The experimental structure factors are subjected to Empirical Potential Structure Refinement (EPSR) modeling to reveal the ion hydration and association and solvent water at the atomic level. About seven water molecules surround Ca at the Ca-O and Ca-D distances of 2.44
at the Ca-O and Ca-D distances of 2.44  and 3.70
and 3.70  , respectively, at both pressures, suggesting no significant pressure effect on the cation hydration. On the other hand, the Cl
, respectively, at both pressures, suggesting no significant pressure effect on the cation hydration. On the other hand, the Cl ion shows a drastic change in water oxygen coordination from 7 at 0.1 MPa to 14 at 1 GPa, accompanied by shortening of Cl-O distance from 3.18
ion shows a drastic change in water oxygen coordination from 7 at 0.1 MPa to 14 at 1 GPa, accompanied by shortening of Cl-O distance from 3.18  to 3.15
to 3.15  . However, the number of water hydrogen atoms around Cl
. However, the number of water hydrogen atoms around Cl does not change significantly as 6.0
does not change significantly as 6.0  6.7 with shortening Cl-D distance from 2.22 to 2.18
6.7 with shortening Cl-D distance from 2.22 to 2.18  on compression. The pressure effect on the solvent water structure is also drastic as an increase in water oxygen atoms of 4.7 at the O-O distance of 2.79
on compression. The pressure effect on the solvent water structure is also drastic as an increase in water oxygen atoms of 4.7 at the O-O distance of 2.79  at 0.1 MPa to 10.3 at 2.85
at 0.1 MPa to 10.3 at 2.85  at 1 GPa. The number of water hydrogen atoms, however, does not change as 1.2 at the O-D distance of 1.74
at 1 GPa. The number of water hydrogen atoms, however, does not change as 1.2 at the O-D distance of 1.74  for both pressures, demonstrating the presence of the O
for both pressures, demonstrating the presence of the O D hydrogen bonds which are significantly bent at 1 GPa at 298 K. This change of hydrogen bonds in water with pressure probably causes the drastic increase in water oxygen atoms around Cl
D hydrogen bonds which are significantly bent at 1 GPa at 298 K. This change of hydrogen bonds in water with pressure probably causes the drastic increase in water oxygen atoms around Cl .
.
 -ray irradiation
-ray irradiationMotooka, Takafumi; Ueno, Fumiyoshi
Zairyo To Kankyo, 64(6), p.220 - 223, 2015/06
Corrosion behavior of carbon steel in chloride aqueous solutions under a low dose rate was investigated by corrosion test using chloride aqueous solutions with different chloride concentration at a dose rate of 500 Gy/h. The corrosion rate of carbon steel increased by the irradiation, and the corrosion rate had the maximum value at a certain chloride concentration. The oxidants produced by radiolysis of chloride aqueous solution enhanced the corrosion of carbon steel. The main oxidants were oxygen and hydrogen peroxide, and the diffusion process of oxidants controlled the corrosion of carbon steel under irradiation. There was a positive correlation between the dependence of corrosion rate and chloride concentration and the dependence of oxidant concentration and chloride concentration.
Arisaka, Makoto; Kimura, Takaumi; Nagaishi, Ryuji; Yoshida, Zenko
Journal of Alloys and Compounds, 408-412, p.1307 - 1311, 2006/02
Times Cited Count:6 Percentile:41.74(Chemistry, Physical)no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
Radiation Research, 163(4), p.455 - 461, 2005/04
Times Cited Count:25 Percentile:56.56(Biology)no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2004-025, TIARA Annual Report 2003, p.141 - 142, 2004/11
no abstracts in English
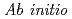 molecular dynamics study of polarization effects on ionic hydration in aqueous AlCl
molecular dynamics study of polarization effects on ionic hydration in aqueous AlCl solution
solutionIkeda, Takashi; Hirata, Masaru; Kimura, Takaumi
Journal of Chemical Physics, 119(23), p.12386 - 12392, 2003/12
Times Cited Count:44 Percentile:78.76(Chemistry, Physical)The solvation shell structure and dynamics of Al and Cl
and Cl in an aqueous solution of 0.8 M AlCl
in an aqueous solution of 0.8 M AlCl are studied under ambient conditions by using
are studied under ambient conditions by using 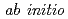 molecular dynamics method. The solvation structures obtained from our
molecular dynamics method. The solvation structures obtained from our 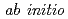 simulations are in good agreement with the experimental ones for both Al
simulations are in good agreement with the experimental ones for both Al and Cl
and Cl . A detailed analysis of intramolecular geometry of hydration waters and dipole moments of the ingredients shows that the polarization has substantial effects on the structures and dynamics of both the cation and anion hydration shells. Implications to the metal hydrolysis of Al
. A detailed analysis of intramolecular geometry of hydration waters and dipole moments of the ingredients shows that the polarization has substantial effects on the structures and dynamics of both the cation and anion hydration shells. Implications to the metal hydrolysis of Al will also be given.
will also be given.
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2003-033, TIARA Annual Report 2002, p.141 - 142, 2003/11
no abstracts in English
 -ray irradiation
-ray irradiationAbe, Yasuhiro*; Takigami, Machiko; Sugino, Koji*; Taguchi, Mitsumasa; Kojima, Takuji; Umemura, Tomonari*; Tsunoda, Kinichi*
Bulletin of the Chemical Society of Japan, 76(8), p.1681 - 1685, 2003/08
Times Cited Count:5 Percentile:26.19(Chemistry, Multidisciplinary)The decomposition of phenolic endocrine disrupting chemicals (P-EDCs), such as phenol, 4-butylphenol (BuP), and bisphenol A (BPA), in aqueous solutions by potassium permanganate (KMnO ) was studied and its efficiency was compared with that of hydroxyl radicals (OH
) was studied and its efficiency was compared with that of hydroxyl radicals (OH ) generated by
) generated by  Co
Co  -ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO
-ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO or OH
or OH . They were formed via direct aromatic ring cleavage in the case of KMnO
. They were formed via direct aromatic ring cleavage in the case of KMnO and OH
and OH addition-substitution reactions followed by aromatic ring cleavage in the case of OH
addition-substitution reactions followed by aromatic ring cleavage in the case of OH . Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO
. Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO spent to completely eliminate BuP and BPA was comparable to the amount of OH
spent to completely eliminate BuP and BPA was comparable to the amount of OH . Although three times more KMnO
. Although three times more KMnO was needed for phenol than OH
was needed for phenol than OH , the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720
, the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720 M of electrons in both cases.
M of electrons in both cases.
Watanabe, Ritsuko; Saito, Kimiaki
Radiation and Environmental Biophysics, 41(3), p.207 - 215, 2002/08
Times Cited Count:21 Percentile:50.70(Biology)no abstracts in English
Nakajima, Ken; Yamane, Yuichi; Ogawa, Kazuhiko; Aizawa, Eiju; Yanagisawa, Hiroshi; Miyoshi, Yoshinori
JAERI-Data/Code 2002-007, 123 Pages, 2002/03
no abstracts in English
Nakajima, Ken; Yamane, Yuichi; Ogawa, Kazuhiko; Aizawa, Eiju; Yanagisawa, Hiroshi; Miyoshi, Yoshinori
JAERI-Data/Code 2002-006, 176 Pages, 2002/03
no abstracts in English
Nakajima, Ken; Yamane, Yuichi; Ogawa, Kazuhiko; Aizawa, Eiju; Yanagisawa, Hiroshi; Miyoshi, Yoshinori
JAERI-Data/Code 2002-005, 158 Pages, 2002/03
no abstracts in English
;
JNC TN8430 2001-006, 65 Pages, 2001/10
We had been conducted to study hydraulic permeability along fracture intersection by NETBLOCK system using natural rock specimen. Since the permeability of this rock specimen fracture is high, it was suggest that turbulent flow might be occurred in available range of measurement system. In case of turbulent flow, estimated permeability and fracture aperture from test data tend to be low. Therefore we should achieve laminar flow. This study was used the high viscosity liquid instead of water, and test conditions which could attain laminar flow with the rock specimen was examined. The rock specimen is granite rock, has natural Y-type fractures intersection. A solution of Methyl-cellulose is used as high viscosity liquid. Due to the high viscosity liquid, hydraulic head could be measured in the wide range, and high viscosity liquid improved the accuracy of measurement. Laminar flow could be achieved in the rock specimen by the high viscosity liquid over 0.1wt%.
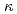 -carrageenan
-carrageenanMakuuchi, Keizo; Yoshii, Fumio; Aranilla, C. T.*; Zhai, M.*
JAERI-Conf 2000-001, p.192 - 195, 2000/03
no abstracts in English
Nagasawa, Naotsugu*; Yoshii, Fumio; Mitomo, Hiroshi*; Kume, Tamikazu
JAERI-Conf 2000-003, p.107 - 119, 2000/03
no abstracts in English