Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
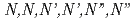 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsubata, Yasuhiro
Solvent Extraction Research and Development, Japan, 31(1), p.1 - 11, 2024/00
A demonstration test was performed to separate minor actinides (MA; Am and Cm) by 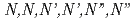 -hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm
-hexaoctyl nitrilotriacetamide (HONTA) as an extractant using mixer-settler extractors installed in a hot cell. A high-level liquid waste containing MA, and rare earths (RE; Y, La, Nd, and Eu) was used as the feed. HONTA diluted to 0.05 mol/dm in
in  -dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were
-dodecane was fed as the organic phase, and a part of the organic phase was reused without solvent regeneration. HONTA effectively extracted MA, whereas RE were less extractable. Consequently, the Y, La, Nd, and Eu ratios distributed to a RE fraction were  99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
99.9%, 99.2%, 61.8%, and 81.4%, respectively. The Am and Cm ratios distributed to an MA fraction were 86.8% and 74.7%, respectively, and a substantial amount of MA (0.12 g) was recovered in the MA fraction by the end of the cumulative duration of 40 h.
Suzuki, Hideya*; Ban, Yasutoshi
Analytical Sciences, 39(8), p.1341 - 1348, 2023/08
Times Cited Count:2 Percentile:67.8(Chemistry, Analytical)The Japan Atomic Energy Agency (JAEA) has proposed the Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation (SELECT) process by solvent extraction as a new separation technology to recover minor actinides (MA) from high-level liquid waste (HLLW) produced by spent fuel reprocessing. The MA separation in the SELECT process comprises the batch recovery of MA and rare earths (RE) from HLLW, MA/RE separation, and Am/Cm separation. Three highly practical extractants are used in the MA separation. Furthermore, this flow configuration facilitates the preparation of nitric acid concentrations in the aqueous phase. However, the separation factor between Cm and Nd in the MA/RE separation is small (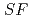
 = 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
= 2.5), requiring many extraction stages for continuous extraction in a mixer-settler. Therefore, this study investigated the separation of only Am from an aqueous nitric acid solution containing MA (Am and Cm) and RE using an organic phase mixed with two extractants alkyl diamideamine with 2-ethylhexyl alkyl chains (ADAAM(EH)) and hexa-n-octylnitrilotriacetamide (HONTA) used in the SELECT process. Under high-concentration nitric acid conditions, Am and La, Ce, Pr, Nd (light lanthanides) were extracted in the ADAAM(EH) + HONTA mixed solvent, whereas Cm, medium, and heavy lanthanides, and Y were partitioned in the aqueous phase. Subsequently, only light lanthanides could be back extracted from the ADAAM(EH) + HONTA mixture solvent containing Am and light lanthanides in low nitric acid concentrations. Furthermore, Am could be easily stripped with 0.2 M or 5 M nitric acid. This method does not require the mutual separation of Cm and Nd, which have low separation factors. Am can be efficiently separated by one extraction and two back-extractions, reducing the number of steps in the SELECT process.
 Tc separation/concentration technology from
Tc separation/concentration technology from  Mo by (n,
Mo by (n,  ) method
) methodFujita, Yoshitaka; Hu, X.*; Takeuchi, Tomoaki; Takeda, Ryoma; Fujihara, Yasuyuki*; Yoshinaga, Hisao*; Hori, Junichi*; Suzuki, Tatsuya*; Suematsu, Hisayuki*; Ide, Hiroshi
KURNS Progress Report 2022, P. 110, 2023/07
no abstracts in English
Nuclear Science and Engineering Center; Fuel Cycle Design Office; Plutonium Fuel Development Center; Nuclear Plant Innovation Promotion Office; Fast Reactor Cycle System Research and Development Center; J-PARC Center
JAEA-Review 2022-052, 342 Pages, 2023/02
This report summarizes the current status and future plans of research and development (R&D) on partitioning and transmutation technology in Japan Atomic Energy Agency, focusing on the results during the 3rd Medium- to Long-term Plan period (FY 2015-2021). Regarding the partitioning technology, R&D of the solvent extraction method and the extraction chromatography method are described, and regarding the minor actinide containing fuel technology, R&D of the oxide fuel production using the simplified pellet method, the nitride fuel production using the external gelation method, and pyrochemical reprocessing of the nitride fuel were summarized. Regarding transmutation technology, R&D of technology using fast reactors and accelerator drive systems were summarized. Finally, the new facilities necessary for the future R&D were mentioned.
Micheau, C.; Ueda, Yuki; Akutsu, Kazuhiro*; Bourgeois, D.*; Motokawa, Ryuhei
Solvent Extraction and Ion Exchange, 41(2), p.221 - 240, 2023/02
Times Cited Count:1 Percentile:39.98(Chemistry, Multidisciplinary)Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Nguyen, T. H.*; Le Ba, T.*; Tran, C. T.*; Nguyen, T. T.*; Doan, T. T. T.*; Do, V. K.; Watanabe, Masayuki; Pham, Q. M.*; Hoang, S. T.*; Nguyen, D. V.*; et al.
Hydrometallurgy, 213, p.105933_1 - 105933_11, 2022/08
Times Cited Count:9 Percentile:80.22(Metallurgy & Metallurgical Engineering)A continuous counter-current extraction for the selective recovery of thorium (Th) and uranium (U) from the Yen Phu (Vietnam) rare earth concentrate leach solutions was systematically studied. The primary amine N1923 was used as an extractant which was prepared in the isoparaffin IP-2028 diluent. Thorium and uranium were selectively recovered in a hydrometallurgical circuit established by continuous mixer-settler extraction, scrubbing, and back-extraction at the laboratory scale. The desired purity of Th and U can be achieved by managing the volume ratio of organic to aqueous phase (O/A ratio) in the corresponding steps. Highly pure Th and U were recovered from the pregnant back-extraction liquor and the raffinate, respectively, which have satisfactory properties for further processing of the subsequent nuclear materials.
Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry Letters (Internet), 13(30), p.7065 - 7071, 2022/08
Times Cited Count:5 Percentile:65.39(Chemistry, Physical) aqueous solution system revealed by fluorescence microspectroscopy
aqueous solution system revealed by fluorescence microspectroscopyMiyagawa, Akihisa*; Kusano, Yuka*; Nagatomo, Shigenori*; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 38(7), p.955 - 961, 2022/07
Times Cited Count:0 Percentile:0(Chemistry, Analytical)In this study, we reveal an Eu(III) extraction mechanism at the interface between HNO and tributyl phosphate (TBP) solutions using fluorescence microspectroscopy. The mass transfer rate constant at the interface is obtained from the analysis of fluorescence intensity changes during the forward and backward extractions at various HNO
and tributyl phosphate (TBP) solutions using fluorescence microspectroscopy. The mass transfer rate constant at the interface is obtained from the analysis of fluorescence intensity changes during the forward and backward extractions at various HNO and TBP concentrations to investigate the reaction mechanism. This result indicates that one nitrate ion reacts with Eu(III) at the interface, whereas TBP molecules are not involved in the interfacial reaction, which is different from the results obtained using the NaNO
and TBP concentrations to investigate the reaction mechanism. This result indicates that one nitrate ion reacts with Eu(III) at the interface, whereas TBP molecules are not involved in the interfacial reaction, which is different from the results obtained using the NaNO solution in our previous study. We demonstrate that the chemical species of Eu(III) complex with nitrate ion and TBP in the aqueous solution play an important role for the extraction mechanism.
solution in our previous study. We demonstrate that the chemical species of Eu(III) complex with nitrate ion and TBP in the aqueous solution play an important role for the extraction mechanism.
Sano, Yuichi; Sakamoto, Atsushi; Miyazaki, Yasunori; Watanabe, So; Morita, Keisuke; Emori, Tatsuya; Ban, Yasutoshi; Arai, Tsuyoshi*; Nakatani, Kiyoharu*; Matsuura, Haruaki*; et al.
Proceedings of International Conference on Nuclear Fuel Cycle; Sustainable Energy Beyond the Pandemic (GLOBAL 2022) (Internet), 4 Pages, 2022/07
We developed a hybrid MA(III) recovery process combining MA(III)+Ln(III) co-recovery flowsheet by solvent extraction with TBP and MA(III)/Ln(III) separation flowsheet by simulated moving bed chromatography using HONTA impregnated adsorbents with large particle size porous silica support.
Ueda, Yuki; Eguchi, Ayano; Tokunaga, Kohei; Kikuchi, Kei*; Sugita, Tsuyoshi; Okamura, Hiroyuki; Naganawa, Hirochika
Industrial & Engineering Chemistry Research, 61(19), p.6640 - 6649, 2022/05
Times Cited Count:1 Percentile:10.7(Engineering, Chemical)no abstracts in English
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:4 Percentile:74.52(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:3 Percentile:38.23(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:1 Percentile:12.61(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
 ]
] from hydrochloric acid using a simple urea extractant
from hydrochloric acid using a simple urea extractantUeda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Wenzel, M.*; Weigand, J. J.*; Oto, Keisuke*
Separation and Purification Technology, 277, p.119456_1 - 119456_8, 2021/12
Times Cited Count:5 Percentile:28.56(Engineering, Chemical)no abstracts in English
Project 6 Meeting Members for Tsukuba International Strategic Zone
JAEA-Review 2021-016, 102 Pages, 2021/11
In December 2011, the Prime Minister designated Tsukuba and some areas in Ibaraki Prefecture as "Comprehensive Special Zones". In the Tsukuba International Strategic Zone, nine advanced research and development (R&D) projects are underway with the goal of promoting industrialization of life innovation and green innovation utilizing the science and technology in Tsukuba. In these projects, the domestic production of medical radioisotope (Technetium-99m,  Tc) was certified as a new project in October 2013, and R&D have been performed in collaboration with related organizations with Japan Atomic Energy Agency (JAEA) as the project leader. Japan is the third largest consumer of molybdenum-99 (
Tc) was certified as a new project in October 2013, and R&D have been performed in collaboration with related organizations with Japan Atomic Energy Agency (JAEA) as the project leader. Japan is the third largest consumer of molybdenum-99 ( Mo) after the United States and Europe, and all
Mo) after the United States and Europe, and all  Mo are imported. Supply will be insufficient if overseas reactors are shut down due to trouble or if transportation (air and land transportations) is stopped due to volcanic eruptions and some accidents. Thus, early domestic production of
Mo are imported. Supply will be insufficient if overseas reactors are shut down due to trouble or if transportation (air and land transportations) is stopped due to volcanic eruptions and some accidents. Thus, early domestic production of  Mo is strongly required. This project is a technology development aimed at domestic production of
Mo is strongly required. This project is a technology development aimed at domestic production of  Mo, which is a raw material of
Mo, which is a raw material of  Tc used as a diagnostic agent. This report summarizes the activities carried out in the first and second phase of the domestic production of medical radioisotope (
Tc used as a diagnostic agent. This report summarizes the activities carried out in the first and second phase of the domestic production of medical radioisotope (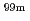 Tc) (here referred to as the "Project 6") in Tsukuba International Strategic Zone (FY2014-2020).
Tc) (here referred to as the "Project 6") in Tsukuba International Strategic Zone (FY2014-2020).
Ueda, Yuki; Morisada, Shintaro*; Kawakita, Hidetaka*; Oto, Keisuke*
Separations (Internet), 8(9), p.139_1 - 139_15, 2021/09
Times Cited Count:9 Percentile:61.52(Chemistry, Analytical)no abstracts in English
 Mo/
Mo/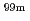 Tc generator by (n,
Tc generator by (n,  ) method, 3
) method, 3Fujita, Yoshitaka; Seki, Misaki; Namekawa, Yoji*; Nishikata, Kaori; Daigo, Fumihisa; Ide, Hiroshi; Tsuchiya, Kunihiko; Sano, Tadafumi*; Fujihara, Yasuyuki*; Hori, Junichi*; et al.
KURNS Progress Report 2020, P. 136, 2021/08
no abstracts in English
Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry B, 125(24), p.6727 - 6731, 2021/06
Times Cited Count:8 Percentile:42.16(Chemistry, Physical)