Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tang, J.*; Wang, Y.*; Fujihara, Hiro*; Shimizu, Kazuyuki*; Hirayama, Kyosuke*; Ebihara, Kenichi; Takeuchi, Akihisa*; Uesugi, Masayuki*; Toda, Hiroyuki*
Scripta Materialia, 239, p.115804_1 - 115804_5, 2024/01
Times Cited Count:0 Percentile:0(Nanoscience & Nanotechnology)Stress corrosion cracking (SCC) behaviors induced by the combination of external and internal hydrogen (H) in an Al-Zn-Mg-Cu alloy were systematically investigated via in situ 3D characterization techniques. SCC of the Al-Zn-Mg-Cu alloy could initiate and propagate in the potential crack region where the H concentration exceeded a critical value, in which the nanoscopic H-induced decohesion of 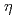 -MgZn
-MgZn precipitates resulted in macroscopic cracking. External H that penetrated the alloy from the environment played a crucial role during the SCC of the Al-Zn-Mg-Cu alloy by generating gradient-distributed H-affected zones near the crack tips, which made Al alloys in water environment more sensitive to SCC. Additionally, the pre-existing internal H was driven toward the crack tips during plastic deformation. It was involved in the SCC and made contributions to both the cracks initiation and propagation.
precipitates resulted in macroscopic cracking. External H that penetrated the alloy from the environment played a crucial role during the SCC of the Al-Zn-Mg-Cu alloy by generating gradient-distributed H-affected zones near the crack tips, which made Al alloys in water environment more sensitive to SCC. Additionally, the pre-existing internal H was driven toward the crack tips during plastic deformation. It was involved in the SCC and made contributions to both the cracks initiation and propagation.
Ao, N.*; Zhang, H.*; Xu, H. H.*; Wu, S. C.*; Liu, D.*; Xu, P. G.; Su, Y. H.; Kang, Q. H.*; Kang, G. Z.*
Engineering Fracture Mechanics, 281, p.109166_1 - 109166_14, 2023/03
Times Cited Count:5 Percentile:89.51(Mechanics)Haoran, W.*; Yu, H.*; Liu, J.*; Kondo, Sosuke*; Okubo, Nariaki; Kasada, Ryuta*
Corrosion Science, 209, p.110818_1 - 110818_12, 2022/12
Times Cited Count:4 Percentile:51.15(Materials Science, Multidisciplinary)The corrosion behavior of newly developed Al O
O forming high Mn oxide dispersion strengthened (ODS) austenitic steels was examined in oxygen-saturated lead-bismuth eutectic at 450
forming high Mn oxide dispersion strengthened (ODS) austenitic steels was examined in oxygen-saturated lead-bismuth eutectic at 450 C for 430 h. Compared with non-ODS steels, the ODS steels possessed superior resistance to corrosion and spallation. The high density grain boundaries in the ODS steels acted as channels for the rapid outward diffusion of metallic elements, forming an internal continuous Cr
C for 430 h. Compared with non-ODS steels, the ODS steels possessed superior resistance to corrosion and spallation. The high density grain boundaries in the ODS steels acted as channels for the rapid outward diffusion of metallic elements, forming an internal continuous Cr O
O scale at the original surface. Accelerated Al diffusion, along with oxidation prevention by the external (Fe, Mn) oxide scale and the internal Cr
scale at the original surface. Accelerated Al diffusion, along with oxidation prevention by the external (Fe, Mn) oxide scale and the internal Cr O
O scale, jointly resulted in the formation of a continuous Al-rich oxide scale in ODS-7Al steel, contributing to its superior corrosion resistance.
scale, jointly resulted in the formation of a continuous Al-rich oxide scale in ODS-7Al steel, contributing to its superior corrosion resistance.
Sato, Tomonori; Hata, Kuniki; Kaji, Yoshiyuki; Taguchi, Mitsumasa*; Seito, Hajime*; Inoue, Hiroyuki*; Tada, Eiji*; Abe, Hiroshi*; Akiyama, Eiji*; Suzuki, Shunichi*
Isotope News, (782), p.40 - 44, 2022/08
The stagnant water in the reactor building at Fukushima Daiichi Nuclear Power Station (1F) is exposed to the radiation from fuel debris and radioactive species. This water contains much amounts of impurities from the seawater which was injected in the emergency cooling. The impurities will affect the radiolysis and corrosive conditions in the water under irradiation. So, the water radiolysis data, corrosion data of steels under irradiations, and the evaluated potential impacts of corrosion in the decommissioning process of 1F are arranged as the database for corrosion under irradiation. This paper introduces the outline of this database.
Yamaguchi, Yoshihito; Mano, Akihiro; Li, Y.
Proceedings of ASME 2022 Pressure Vessels and Piping Conference (PVP 2022) (Internet), 10 Pages, 2022/07
The steam generator (SG) tube is one of the important components in pressurized water reactors. Flaws such as wall-thinning or stress corrosion cracking have been reported in SG tubes. The burst pressure where both the internal and external pressures from the primary and secondary coolant systems are considered must be predicted to assess the structural integrity of SG tubes. Burst tests were performed by various organizations. On the basis of the test results, failure estimation methods were proposed. In this study, previous burst test data and existing failure estimation methods for SG tubes with wall-thinning or crack were investigated. As a result, the coefficient of the existing estimation method for SG tube with uniform wall-thinning was updated. In addition, failure estimation methods that are suitable for SG tubes with crack or local wall-thinning were proposed by considering the effects of the flaw shape and size on the burst pressure. The applicability of the failure estimation methods was confirmed by comparing the predicted results with the burst test data in actual SG tubes.
Soma, Yasutaka; Komatsu, Atsushi; Ueno, Fumiyoshi
Corrosion, 78(6), p.503 - 515, 2022/06
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary)The effects of electrochemical potential (ECP) on water chemistry within a crevice are of critical importance for understanding stress corrosion cracking (SCC) of Fe-Cr-Ni alloys in high temperature water. In this study, the effects of ECP on the electrical conductivity of a solution within a Type-316L stainless steel crevice (
 ) have been studied in 288
) have been studied in 288 C and 8 MPa water containing 10 ppb Cl
C and 8 MPa water containing 10 ppb Cl as major anionic species. In situ measurements of
as major anionic species. In situ measurements of 
 in a rectangular crevice with a gap of 15
in a rectangular crevice with a gap of 15  m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in
m and a depth of 23 mm have been conducted using small sensors installed at different crevice depths. An increase in ECP from -0.49 V (vs. standard hydrogen electrode) to -0.12 V resulted in an increase in 
 from 12
from 12  Scm
Scm to 160
to 160  Scm
Scm at a distance of 21 mm from the crevice mouth. The increase in
at a distance of 21 mm from the crevice mouth. The increase in 
 reached a maximum at about 0.15 V (about 300
reached a maximum at about 0.15 V (about 300  Scm
Scm ) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl
) and then tended to decrease with increasing potential. Finite element model analysis taking into account the electrochemical reaction quantitatively reproduced this behavior. It is considered that Cl is the major anionic species transported into the crevice at relatively low potentials, and that
is the major anionic species transported into the crevice at relatively low potentials, and that 
 increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
increases monotonically with increasing ECP. On the other hand, when ECP exceeds around 0 V, a sufficient amount of HCrO
 generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
generated by transpassive dissolution also transported into the gap. Since this chemical species is highly oxidizing, unlike Cl, it is assumed that it reacts with metal cations to oxidize and precipitate them, thereby lowering conductivity.
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.
Igarashi, Takahiro; Otani, Kyohei; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Bosei Kanri, 66(4), p.141 - 145, 2022/04
Metal corrosion is a material deterioration phenomenon based on electrochemical reactions on an atomic scale. In this paper, various methods for acquiring physical properties on metal surfaces using first-principles calculations were described. As examples of applying first-principles calculation to metal corrosion, the effect of hydrogen adsorption on the metal surface on the potential change and the effect of cation atoms in the aqueous solution on the corrosion resistance of the metal were reported.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Materials Transactions, 63(4), p.538 - 544, 2022/04
Times Cited Count:0 Percentile:0(Materials Science, Multidisciplinary)The time dependence of the corrosion behavior of tantalum (Ta), which is used in nuclear fuel reprocessing equipment, in sodium hydroxide (NaOH) solutions was investigated by immersion tests, and the mechanism of the time dependence was examined via surface observations and electrochemical measurements. The immersion tests were conducted at room temperature with NaOH concentrations ranging from 1 to 7 mol/L for immersion periods of 24 to 168 h. The corrosion rate increased with the NaOH concentration but peaked with the immersion period and then decreased. The time to peak of the corrosion rate was shorter with higher NaOH concentration. The X-ray diffraction (XRD) patterns and Raman spectra of the surfaces of the specimens immersed in the 7 mol/L NaOH solution for more than 48 h showed Na Ta
Ta O
O formation. The polarization resistance decreased with immersion time for all NaOH concentrations up to about 24 h after immersion. Thereafter, the polarization resistance increased with immersion time in 7 mol/L NaOH solution and remained almost constant in the other NaOH concentrations. Findings suggested that the change in the corrosion rate was affected by the film formation during immersion, since the time dependence of the polarization resistance and the sum of film resistance and charge transfer resistance had the same tendencies. The precipitation film was mainly Na
formation. The polarization resistance decreased with immersion time for all NaOH concentrations up to about 24 h after immersion. Thereafter, the polarization resistance increased with immersion time in 7 mol/L NaOH solution and remained almost constant in the other NaOH concentrations. Findings suggested that the change in the corrosion rate was affected by the film formation during immersion, since the time dependence of the polarization resistance and the sum of film resistance and charge transfer resistance had the same tendencies. The precipitation film was mainly Na Ta
Ta O
O formed by the dissolution of the passivity film on Ta.
formed by the dissolution of the passivity film on Ta.
Saito, Shigeru; Wan, T.*; Okubo, Nariaki; Kita, Satoshi*; Obayashi, Hironari; Sasa, Toshinobu
JAEA-Technology 2021-034, 94 Pages, 2022/03
Lead-bismuth eutectic alloy (LBE) is a major candidate for a spallation target material and core coolant of an accelerator driven system (ADS) which has been developed in the Japan Atomic Energy Agency (JAEA) to transmute high-level radioactive wastes. A proton irradiation facility to build a material irradiation database for future ADS development is under considering in the J-PARC. To realize both the ADS and the above-mentioned facility, there are many issues on operational safety of LBE to be solved. Especially, corrosion data for the major materials such as T91 (Modified 9Cr-1Mo steel) and SS316L at the temperature range between 400 and 550  C under the conditions of flowing LBE with a controlled oxygen are not sufficient to design the ADS and the facility. JAEA developed a new large-scale corrosion test loop named "OLLOCHI (Oxygen-controlled LBE LOop for Corrosion tests in HIgh-temperature)" aiming to perform the compatibility tests between the LBE and the steels, as well as to develop the LBE operation technology. OLLOCHI has a function to automatically control the oxygen concentration in LBE. The maximum temperature at the regions of high-temperature and low-temperature of the OLLOCHI are 550
C under the conditions of flowing LBE with a controlled oxygen are not sufficient to design the ADS and the facility. JAEA developed a new large-scale corrosion test loop named "OLLOCHI (Oxygen-controlled LBE LOop for Corrosion tests in HIgh-temperature)" aiming to perform the compatibility tests between the LBE and the steels, as well as to develop the LBE operation technology. OLLOCHI has a function to automatically control the oxygen concentration in LBE. The maximum temperature at the regions of high-temperature and low-temperature of the OLLOCHI are 550  C and 450
C and 450  C respectively to cover the ADS designed condition. As a result of 2,000 hours operation, it was demonstrated that the OLLOCHI showed the designed performance. In this report, outline of the OLLOCHI, details of the components, results of characteristic tests, and the future experimental plan are described.
C respectively to cover the ADS designed condition. As a result of 2,000 hours operation, it was demonstrated that the OLLOCHI showed the designed performance. In this report, outline of the OLLOCHI, details of the components, results of characteristic tests, and the future experimental plan are described.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
Zairyo To Kankyo, 71(2), p.40 - 45, 2022/02
The purpose of this study is to investigate the effect of oxygen concentration in the air on the corrosion rate of carbon steel in an air/solution alternating environment in the low oxygen concentration range and to clarify the corrosion rate and corrosion mechanism of carbon steel depending on the oxygen concentration in air by the mass change of specimens before and after the corrosion test and observing the iron rust layer formed on the surface of carbon steel. The corrosion rate increases with increasing oxygen concentration in the air, and the gradient of the corrosion rate decreases gradually. The maximum erosion depth increased with increasing oxygen concentration except for the case of 1% oxygen concentration, however, the maximum erosion depth for 1% oxygen concentration was larger than that for 5% air oxygen concentration.
Sasa, Toshinobu
Kasokuki, 18(4), p.233 - 240, 2022/01
Lead bismuth eutectic alloy (LBE) is a promising option as a spallation target for accelerator-driven transmutation systems (ADS) to reduce the radiological toxicity from long-lived radioactive waste. LBE is a heavy metal and has suitable characteristics both as a spallation target and as a coolant for transmutation systems. However, LBE is also known as a highly corrosive with structural materials. In this paper, technological developments to overcome the issue, the latest research activities such as hightemperature operation and oxygen concentration control to ensure corrosion resistance, are introduced together with the outline of the target for ADS.
Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Tetsu To Hagane, 107(12), p.998 - 1003, 2021/12
Times Cited Count:0 Percentile:0(Metallurgy & Metallurgical Engineering)In order to clarify the effect of environmental factors on the amount of atmospheric corrosion of steel, novel model for predicting the reduction of atmospheric corrosion considering relative humidity and rain falls was developed. We conducted a one-year calculation simulation of atmospheric corrosion in Miyakojima City, Choshi City, and Tsukuba City using the developed model. Corrosion weight loss by the simulation could reproduce the measured value well. Corrosion weight loss at each point was greatly affected by the amount of flying sea salt, relative humidity, and rain falls.
Otani, Kyohei; Kato, Chiaki
Zairyo To Kankyo, 70(12), p.480 - 486, 2021/12
This is a comprehensive paper of the corrosion of carbon steel in air/solution alternating condition. From cross-sectional observation and analysis of the iron rust layer formed on the surface of carbon steel in the alternating condition, it was found that a multilayered iron rust layer composed of red rust layer ( -FeOOH), rust crust layer (Fe
-FeOOH), rust crust layer (Fe O
O ), inner crystal (Fe
), inner crystal (Fe O
O ), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
), and inner rust layer was formed on carbon steel. The multi-layered iron rust layer would accelerate the cathodic oxygen reduction reaction, and the reason why the corrosion rate of the carbon steel in the alternating condition was accelerated. The effect of artificial seawater (ASW) composition on the corrosion rate of carbon steel in air/solution alternating condition was investigated. It was found that the corrosion rate increased with increasing concentration from pure water to 200 times diluted ASW, and decreased with increasing concentration from 20 times diluted ASW to no diluted ASW. The Mg and Ca ions in ASW precipitated on the reaction interface and formed a metal cation layer, which inhibited the oxygen reduction reaction, and thus the corrosion of carbon steel was inhibited in the highly concentrated ASW.
Hata, Kuniki
Zairyo To Kankyo, 70(12), p.468 - 473, 2021/12
In order to estimate corrosive environment in the contaminated water at Fukushima Daiichi Nuclear Power Station, effects of oxidants, such as H O
O , which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and
, which were generated from water radiolysis, should be taken into account due to the irradiation field in the reactor building. The process of water radiolysis and the amounts of these oxidants can change depending on the conditions of water and types of radiation. After the accident, a variety of factors, which can affect water radiolysis, such as seawater constituents, surface of oxides, and  -radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
-radionuclides, had been discussed. In this paper, these effects on radiolysis are reviewed for the better understanding of the corrosive environment in the contaminated water.
Wakai, Satoshi*; Hirano, Shinichi*; Ueno, Fumiyoshi; Okamoto, Akihiro*
Zairyo To Kankyo, 70(12), p.491 - 496, 2021/12
After Fukushima Daiichi Nuclear Power Station accident, various corrosion mitigating activities have been treated, and severe corrosion incident have never taken placed. On the other hand, the facilities were exposed sea water, and some of them have continuously exposed to ground water. The exposure of metal materials to environmental water has a risk of microbiologically influenced corrosion (MIC). In this paper, we summarize the latest knowledge of MIC and the task of MIC in the decommissioning of Fukushima Daiichi Nuclear Power Station.
Igarashi, Takahiro; Komatsu, Atsushi; Kato, Chiaki; Sakairi, Masatoshi*
Bosei Kanri, 65(10), p.365 - 370, 2021/10
We have developed a new atmospheric simulation model considering important environmental factors such as airborne sea salt, temperature, relative humidity, and rainfall. The developed model was verified by comparing predicted values by the simulation and measured data for the weight loss by atmospheric corrosion. In addition, atmospheric corrosion simulations under open and sheltered exposure condition were conducted, and it was confirmed that the air corrosion weight loss was strongly suppressed by the surface cleaning effect due to rainfall.
Sato, Tomonori; Hata, Kuniki; Kaji, Yoshiyuki; Ueno, Fumiyoshi; Inoue, Hiroyuki*; Taguchi, Mitsumasa*; Seito, Hajime*; Tada, Eiji*; Abe, Hiroshi*; Akiyama, Eiji*; et al.
JAEA-Review 2021-001, 123 Pages, 2021/06
In the implement of the decommissioning of Fukushima Daiichi Nuclear Power Station (1F), there are many problems to be solved. Specially, the mitigation of the aging degradation by the corrosion of the structural materials is important to implement the decommissioning safely and continuously. However, there are limited data for the environmental factors of corrosion in 1F, and the condition of 1F is continuously changing. So, the literature data for the water radiolysis and the corrosion under irradiation are listed as the database of corrosion under irradiation in this report. And the new obtained radiolysis and corrosion data, which have not been reported in the literature and will be required in the decommissioning of 1F, are reported.
Ishijima, Yasuhiro; Ueno, Fumiyoshi; Abe, Hitoshi
Zairyo To Kankyo, 70(6), p.192 - 198, 2021/06
The time dependence of corrosion behavior on tantalum used in nuclear fuel reprocessing equipment in sodium hydroxide solution was investigated by immersion corrosion tests, and the mechanism of aging change was discussed from surface observations and electrochemical measurements. The immersion tests were carried out at room temperature with NaOH concentrations ranging from 1 to 7 mol/L and immersion times ranging from 24 to 168 hr, respectively. The corrosion rate increased with NaOH concentration, but peaked with immersion time and then decreased. The time to peak of corrosion rate was shorter with higher NaOH concentration. The SEM observations and Raman analysis at the surface of the specimens that were cleaned and weighed after the immersion test did not show any film formation. On the other hand, the polarization resistance showed a constant value or an increase after a decrease immediately after immersion. It is suggested that the change in corrosion rate is affected by the formation of film by immersion, since the value of polarization resistance is almost the same as the sum of film resistance and charge transfer resistance. The film was considered to be mainly Na Ta
Ta O
O formed by the dissolution of Ta.
formed by the dissolution of Ta.
Kano, Koichi*; Hagiwara, Satoshi*; Igarashi, Takahiro; Otani, Minoru*
Electrochimica Acta, 377, p.138121_1 - 138121_10, 2021/05
Times Cited Count:17 Percentile:68.84(Electrochemistry)We investigated the free corrosion potential at an interface between an Al electrode and an aqueous NaCl solution under acidic conditions via density functional theory combined with the effective screening medium and reference interaction site model (ESM-RISM). The electrode potentials for the anodic and cathodic corrosion reactions were obtained from the grand potential profile as a function of the electron chemical potential at the interface. Thereafter, we determined the free corrosion potential using the Tafel extrapolation method. The results of the free corrosion potential were consistent with previous experimental data. By controlling the pH, we determined the pH dependence of the free corrosion potential, and the results agreed well with the experimental results. Our results indicated that the ESM-RISM method duly described the environmental effect of an acidic solution and precisely determined the free corrosion potential.