Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -CsOH
-CsOHRizaal, M.; Luu, V. N.; 中島 邦久; 三輪 周平
Proceedings of International Topical Workshop on Fukushima Decommissioning Research 2024 (FDR2024) (Internet), 4 Pages, 2024/10
Thermochemistry prevailing between gaseous CsOH and concrete main chemical phase CaCO at temperatures up to 570
at temperatures up to 570 C was investigated with various scenarios using the thermogravimetric method. The aim was to elucidate the decreasing behavior of cesium (Cs) trapping on CaCO
C was investigated with various scenarios using the thermogravimetric method. The aim was to elucidate the decreasing behavior of cesium (Cs) trapping on CaCO observed in the transpiration method. A quasi-two-compartment platinum crucible was developed to realize co-measurements of both CsOH and CaCO
observed in the transpiration method. A quasi-two-compartment platinum crucible was developed to realize co-measurements of both CsOH and CaCO during thermal treatment. Post-test X-ray diffraction was conducted to identify the chemical compound formed on the CaCO
during thermal treatment. Post-test X-ray diffraction was conducted to identify the chemical compound formed on the CaCO precursor. The early presence (timely sensitivity) of CsOH near the heated surface of CaCO
precursor. The early presence (timely sensitivity) of CsOH near the heated surface of CaCO was found to play a key role in the trapping (in the form of Cs
was found to play a key role in the trapping (in the form of Cs CO
CO ). Such a factor is crucial because, otherwise, the Ca(OH)
). Such a factor is crucial because, otherwise, the Ca(OH) would predominate the surface upon CaCO
would predominate the surface upon CaCO decomposition where leading to no reaction with CsOH.
decomposition where leading to no reaction with CsOH.
 at temperature range 170 - 290
at temperature range 170 - 290 C
CLuu, V. N.; 中島 邦久
Nuclear Engineering and Design, 426, p.113402_1 - 113402_7, 2024/09
被引用回数:0 パーセンタイル:0.00(Nuclear Science & Technology)A field assessment at the Fukushima-Daiichi Nuclear Power Station revealed high radioactivity on the concrete shield plugs, which is estimated above 20 PBq for Cs-137 at units 2 and 3. This leads to significant interest in the retention of Cs on concrete during severe accidents (SA). However, the interaction of CsOH, as one of the main Cs forms released in SA, with concrete surfaces at elevated temperatures remains poorly researched. In this study, we have experimentally investigated the deposition behavior of CsOH on CaCO , which is the primary phase existing on the surface of concrete, under humid atmosphere. As a result, the chemical reaction enhanced deposition rate (N), and increased linearly with CsOH concentration (C
, which is the primary phase existing on the surface of concrete, under humid atmosphere. As a result, the chemical reaction enhanced deposition rate (N), and increased linearly with CsOH concentration (C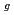 ), as following expression: N(
), as following expression: N( g/cm
g/cm
 s) = v
s) = v C
C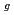 , where v
, where v is temperature-dependent deposition velocity as given by ln v
is temperature-dependent deposition velocity as given by ln v (cm/s) = -3785.8/T + 3.766, for T in the range of 170 and 290
(cm/s) = -3785.8/T + 3.766, for T in the range of 170 and 290  C. This empirical model can be integrated into severe accident codes to quantify the chemical trapping of cesium on concrete surfaces during ex-vessel release. Moreover, it can contribute to understanding the reasons behind the high dose rate on concrete shield plugs at the Fukushima Daiichi Nuclear power stations and aid in developing effective decommissioning practices for concrete structures.
C. This empirical model can be integrated into severe accident codes to quantify the chemical trapping of cesium on concrete surfaces during ex-vessel release. Moreover, it can contribute to understanding the reasons behind the high dose rate on concrete shield plugs at the Fukushima Daiichi Nuclear power stations and aid in developing effective decommissioning practices for concrete structures.
 C
CLuu, V. N.; 中島 邦久
Mechanical Engineering Journal (Internet), 11(2), p.23-00446_1 - 23-00446_11, 2024/01
Cesium distribution is crucial for decommissioning Fukushima Daiichi Nuclear Power Station (1F). Several experimental studies confirmed Cs retention on stainless steels by performing chemical reactions at high temperatures (typically above 800 C)), but the Cs retention on concrete, used in large quantities in light water reactors, is not fully understood. This study demonstrated that Cs might have been deposited and retained on the concrete structures where the temperature was not so high during the 1F accident. Results showed that the CsOH/concrete interaction at around 200
C)), but the Cs retention on concrete, used in large quantities in light water reactors, is not fully understood. This study demonstrated that Cs might have been deposited and retained on the concrete structures where the temperature was not so high during the 1F accident. Results showed that the CsOH/concrete interaction at around 200 C occurred in water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO
C occurred in water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO to form Cs
to form Cs CO
CO hydrate, and with aluminosilicate and SiO
hydrate, and with aluminosilicate and SiO (quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output could help elucidate the trapping mechanism that caused extremely high radioactivity on concrete shield plugs at 1F and develop an effective decommissioning practice for concrete structures.
(quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output could help elucidate the trapping mechanism that caused extremely high radioactivity on concrete shield plugs at 1F and develop an effective decommissioning practice for concrete structures.
 C
CLuu, V. N.; 中島 邦久
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 9 Pages, 2023/05
Information of Cs distribution is important for decommissioning of the Fukushima Daiichi Nuclear Power Station (1F). Several experimental studies confirmed the Cs retention on stainless steels by chemical reaction at very high temperatures (commonly above 800 C), but the Cs retention on non-metallic materials, such as concrete and thermal insulators, was not fully understood though they are used with large quantity in light water reactors. This study demonstrated that Cs might be deposited and retained on the concrete structure where the temperature was not so high during the 1F accident. It was revealed that the CsOH/concrete interaction at around 200
C), but the Cs retention on non-metallic materials, such as concrete and thermal insulators, was not fully understood though they are used with large quantity in light water reactors. This study demonstrated that Cs might be deposited and retained on the concrete structure where the temperature was not so high during the 1F accident. It was revealed that the CsOH/concrete interaction at around 200 C resulted in the formation of water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate, if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO
C resulted in the formation of water-insoluble Cs-(Al,Fe)-Si-O deposits and water-soluble phases, i.e., cesium carbonate hydrate and possibly cesium silicate, if Al and Fe are not present. CsOH might be trapped on concrete by chemical reaction with CaCO to form Cs
to form Cs CO
CO hydrate, and with aluminosilicate and SiO
hydrate, and with aluminosilicate and SiO (quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output will be useful for elucidating the trapping mechanism that caused an extremely high radioactivity on concrete shield plugs at 1F, and for developing an effective decommissioning practice for concrete structure.
(quartz) to form Cs-Al-Si-O and Cs-Si-O deposits, respectively. This output will be useful for elucidating the trapping mechanism that caused an extremely high radioactivity on concrete shield plugs at 1F, and for developing an effective decommissioning practice for concrete structure.
Luu, V. N.; 中島 邦久
Journal of Nuclear Science and Technology, 60(2), p.153 - 164, 2023/02
被引用回数:6 パーセンタイル:68.73(Nuclear Science & Technology)Recently, extremely high dose rates were detected in the three-layer concrete plugs of Units 2 and 3 at the Fukushima Daiichi Nuclear Power Plant. The high dose rates suggest that there are some trapping effects of radioactive materials on shield plugs when gas species and aerosols (e.g., CsOH, CsI) are released from reactor through the plug layers. To determine the trapping mechanism, concrete and commonly used aggregate and minerals are pulverized and mixed with CsOH, followed by heating at different temperatures to clarify the chemical interaction. The results showed that interactions of CsOH and CaCO in concrete occurred even at room temperature to form Cs
in concrete occurred even at room temperature to form Cs CO
CO (H
(H O)
O) . The interaction with aggregates occurred above 100
. The interaction with aggregates occurred above 100 C and resulted in the formation of CsAlSiO
C and resulted in the formation of CsAlSiO . Additionally, amorphous and crystalline SiO
. Additionally, amorphous and crystalline SiO interacted with CsOH, forming a glass-like product above 200
interacted with CsOH, forming a glass-like product above 200 C. These results suggest that formation of Cs
C. These results suggest that formation of Cs CO
CO (H
(H O)
O) would be one of the main trapping mechanism at shield plugs because CaCO
would be one of the main trapping mechanism at shield plugs because CaCO is commonly formed on concrete surface and reacts with CsOH at room temperature.
is commonly formed on concrete surface and reacts with CsOH at room temperature.
伊勢 英夫*; 伊崎 誠*; 大石 晴夫*; 森 清治*; 阿向 賢太郎*; 森山 尚*; 加賀谷 博昭*; 小林 正巳*; 田口 浩*; 柴沼 清
FAPIG, (159), p.10 - 14, 2001/11
国際熱核融合実験炉(ITER)のブランケット等の炉内構造機器を保守する際、水平ポート部に設置された約40tonの遮蔽プラグ等(以下、プラグ)を事前に取り扱う必要がある。その際、遠隔装置による片持ち取扱いが設計条件となる。本稿では実機装置の設計,縮小(1/2.5)モデル製作及び試験結果について報告する。設計では、プラグの最終位置決め精度( 1mm)を満足できるよう、走行,把持フックの独立昇降,フック取付アームチルトの4自由度のほか,トロイダル方向の相対位置ずれ(
1mm)を満足できるよう、走行,把持フックの独立昇降,フック取付アームチルトの4自由度のほか,トロイダル方向の相対位置ずれ( 5mm)に対処可能なように受動的コンプライアンス機構を備えた装置とした。縮小モデル制御システムの設計でき、遠隔操作部PCのOSにRT-Linuxを採用してリアルタイムプロセスが実行可能とした。縮小モデルを用いた理想状態(
5mm)に対処可能なように受動的コンプライアンス機構を備えた装置とした。縮小モデル制御システムの設計でき、遠隔操作部PCのOSにRT-Linuxを採用してリアルタイムプロセスが実行可能とした。縮小モデルを用いた理想状態( 1mm以下の位置ずれ量)での基本試験では、再現性の良いプラグ着脱手順,各軸座標,荷重条件等が明確となり、本装置の機構により片持ち方式による実機プラグの取扱いが可能である見通しを得た。
1mm以下の位置ずれ量)での基本試験では、再現性の良いプラグ着脱手順,各軸座標,荷重条件等が明確となり、本装置の機構により片持ち方式による実機プラグの取扱いが可能である見通しを得た。
 C
CLuu, V. N.; 中島 邦久
no journal, ,
Chemisorption tests of CsOH onto concrete and aggregate were carried out at 200 C in both dry and humid conditions to investigate the source of high dose rates at the concrete shield plugs of Units 2 and 3 at 1F. Post-test analyses using XRD, Raman, and SEM revealed that water-insoluble Cs-bearing deposits formed different morphologies under dry and humid conditions. Namely, uniform and large Cs deposits were formed under humid conditions, while heterogenous growth and smaller size Cs deposits were observed under dry conditions.
C in both dry and humid conditions to investigate the source of high dose rates at the concrete shield plugs of Units 2 and 3 at 1F. Post-test analyses using XRD, Raman, and SEM revealed that water-insoluble Cs-bearing deposits formed different morphologies under dry and humid conditions. Namely, uniform and large Cs deposits were formed under humid conditions, while heterogenous growth and smaller size Cs deposits were observed under dry conditions.
 at temperature range 170 - 290
at temperature range 170 - 290  C
CLuu, V. N.; 中島 邦久
no journal, ,
A recent field survey revealed extremely high radioactivity on the concrete shield plugs, exceeding 20 PBq for Cs-137 at units 2, 3 of 1F. This leads to significant interest in the retention of Cs on concrete during severe accident. Despite this, the interaction of Cs vapors and/or aerosols in the gas phase with concrete surfaces at high temperatures remains inadequately explored. In this work, we investigated the deposition behavior of CsOH on CaCO , as a primary phase on the concrete surface, under humid atmosphere. As a result, the chemical reaction enhanced deposition rate, and increased linearly with CsOH concentration.
, as a primary phase on the concrete surface, under humid atmosphere. As a result, the chemical reaction enhanced deposition rate, and increased linearly with CsOH concentration.
Luu, V. N.; 中島 邦久
no journal, ,
This study investigated the possibility of chemical interaction between concrete/aggregate and cesium hydroxide at high temperatures. CsOH interaction with CaCO in concrete forms water-soluble Cs
in concrete forms water-soluble Cs CO
CO (H
(H O)
O) even at room temperature, whereas CsOH interaction with aluminosilicate minerals in aggregate forms water-insoluble CsAlSiO
even at room temperature, whereas CsOH interaction with aluminosilicate minerals in aggregate forms water-insoluble CsAlSiO just above 100
just above 100 C, according to TG/DTA and XRD analyses on the mixture of CsOH and pulverized concrete/aggregate. The results suggest that the formation of Cs
C, according to TG/DTA and XRD analyses on the mixture of CsOH and pulverized concrete/aggregate. The results suggest that the formation of Cs CO
CO (H
(H O)
O) would be one of the main trapping mechanisms at shield plugs because CaCO
would be one of the main trapping mechanisms at shield plugs because CaCO is commonly formed on concrete surfaces and reacts with CsOH at room temperature.
is commonly formed on concrete surfaces and reacts with CsOH at room temperature.
Luu, V. N.; 中島 邦久
no journal, ,
To investigate the source of high dose rates at the concrete shield plugs of Unit 2 and 3 at 1F, high temperature tests on the mixture of CsOH and pulverized concrete/main components were conducted. Results showed that both water-soluble and -insoluble phases were formed below 300 C. Namely, Cs
C. Namely, Cs CO
CO (H
(H O)
O) was formed due to chemical reaction with CaCO
was formed due to chemical reaction with CaCO at room temperature. The authors will discuss the possibility that this might be one of the main trapping mechanisms on shield plugs.
at room temperature. The authors will discuss the possibility that this might be one of the main trapping mechanisms on shield plugs.