Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Mochizuki, Akihito; Matsui, Hiroya; Nakayama, Masashi; Sakamoto, Ryo*; Shibata, Masahito*; Motoshima, Takayuki*; Jo, Mayumi*
Case Studies in Construction Materials, 22, p.e04648_1 - e04648_20, 2025/07
Times Cited Count:0The properties of low-pH cement used in the geological disposal of radioactive waste may change through atmospheric carbonation and degradation caused by groundwater during the long-term operation of a repository. In this study, we investigated the effects of atmospheric carbonation and groundwater contact on the chemical, microstructural, and transport properties of shotcrete made from low-pH, high-fly-ash silica-fume cement (HFSC) over a period of 16 years in an underground research laboratory. In both carbonated and degraded zones of the HFSC shotcrete, capillary porosity increased for pores of  300 nm in diameter, and the total porosity was higher than in undegraded zones. These changes in porosity may be associated with the decalcification of calcium-silicate-hydrate and decomposition of ettringite. Such changes were minor in altered zones of OPC shotcrete, indicating that HFSC shotcrete is less resistant to atmospheric carbonation and groundwater leaching under the studied conditions. However, the hydraulic conductivity in HFSC was low enough to fulfill the specific functional requirements of low-pH cements for geological disposal.
300 nm in diameter, and the total porosity was higher than in undegraded zones. These changes in porosity may be associated with the decalcification of calcium-silicate-hydrate and decomposition of ettringite. Such changes were minor in altered zones of OPC shotcrete, indicating that HFSC shotcrete is less resistant to atmospheric carbonation and groundwater leaching under the studied conditions. However, the hydraulic conductivity in HFSC was low enough to fulfill the specific functional requirements of low-pH cements for geological disposal.
Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Steinle-Neumann, G.*; Hattori, Takanori; Yuan, L.*; Suzuki, Akio*
Journal of Mineralogical and Petrological Sciences (Internet), 120(1), p.240926a_1 - 240926a_13, 2025/06
We explore the structures of dry and hydrated (H O and D
O and D O) Na
O) Na Si
Si O
O melt at 0-6 GPa and 1000-1300 K and glasses recovered from high pressure and temperatures by in-situ neutron and X-ray diffraction. The structures of the melts at 0-10 GPa and 3000 K are also investigated by ab-initio molecular dynamics simulation. In-situ neutron experiments revealed that the D-O distance increases with compression due to the formation of -O-D-O- bridging species, which is reproduced by the molecular dynamics simulations. The pressure-induced -O-D-O- formation reflects a more rigid incorporation of hydrogen, which acts as a mechanism for the experimentally observed higher solubility of water in silicate melts. Together with shrinking modifier domains, this process dominates the compression behavior of hydrous Na
melt at 0-6 GPa and 1000-1300 K and glasses recovered from high pressure and temperatures by in-situ neutron and X-ray diffraction. The structures of the melts at 0-10 GPa and 3000 K are also investigated by ab-initio molecular dynamics simulation. In-situ neutron experiments revealed that the D-O distance increases with compression due to the formation of -O-D-O- bridging species, which is reproduced by the molecular dynamics simulations. The pressure-induced -O-D-O- formation reflects a more rigid incorporation of hydrogen, which acts as a mechanism for the experimentally observed higher solubility of water in silicate melts. Together with shrinking modifier domains, this process dominates the compression behavior of hydrous Na Si
Si O
O melt, whereas the compression of dry Na
melt, whereas the compression of dry Na Si
Si O
O at 0-10 GPa and 3000 K is governed largely by bending of the Si-O-Si angle. The molecular dynamics simulations on hydrous Na
at 0-10 GPa and 3000 K is governed largely by bending of the Si-O-Si angle. The molecular dynamics simulations on hydrous Na Si
Si O
O melts further suggest that the sodium ions are scavenged from its network-modifying role via 2(
melts further suggest that the sodium ions are scavenged from its network-modifying role via 2(![$$^{[4]}$$](/search/images/ERPHWWZULV4SI.gif) Si-O
Si-O + Na
+ Na )
) 
![$$^{[4]}$$](/search/images/ERPHWWZULV4SI.gif) Si-(O-
Si-(O-![$$^{[5]}$$](/search/images/ERPHWWZVLV4SI.gif) Si-O)
Si-O) + 2Na
+ 2Na and Si-O
and Si-O + Na
+ Na + Si-OH
+ Si-OH  Si-(O-H-O-Si)
Si-(O-H-O-Si) + Na
+ Na with increasing pressure.
with increasing pressure.
Takahatake, Yoko; Watanabe, So; Watanabe, Masayuki; Sano, Yuichi; Takeuchi, Masayuki
Progress in Nuclear Science and Technology (Internet), 7, p.195 - 198, 2025/05
Extraction chromatgraphy technology for trivalent minor actinide (MA(III) ; Am(III) and Cm(III)) recovery from the solution generated by an extraction process in reprocessing of spent nuclear fuel has been developed. A fine particle is generated in the solution. The fine particle must be removed before MA recovery operation, because that leads clogging of the extraction chlomatography column. In order to prevent clogging the column, filtration system utilizing porous silica beads packed column has been designed. In this study, a fine particle trapping system was developed and particle removal performance of the system was experimentally evaluated using alumina particles as simulated fine particle. Column experiments revealed that the fine particle with the particle size from 0.12 to 15  m is cause of clogging of the filtration column. Since simulated fine particles were trapped on filtration experiments, a filtration system using the porous silica beads column is practical,
m is cause of clogging of the filtration column. Since simulated fine particles were trapped on filtration experiments, a filtration system using the porous silica beads column is practical,
 hydridosilicate at high pressures; A Bridge to BaSiH
hydridosilicate at high pressures; A Bridge to BaSiH polyhydride
polyhydrideBeyer, D. C.*; Spektor, K.*; Vekilova, O. Y.*; Grins, J.*; Barros Brant Carvalho, P. H.*; Leinbach, L. J.*; Sannemo-Targama, M.*; Bhat, S.*; Baran, V.*; Etter, M.*; et al.
ACS Omega (Internet), 10(15), p.15029 - 15035, 2025/04
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)Hydridosilicates featuring SiH octahedral moieties represent a rather new class of compounds with potential properties relating to hydrogen storage and hydride ion conductivity. Here, we report on the new representative BaSiH
octahedral moieties represent a rather new class of compounds with potential properties relating to hydrogen storage and hydride ion conductivity. Here, we report on the new representative BaSiH obtained from reacting the Zintl phase hydride BaSiH
obtained from reacting the Zintl phase hydride BaSiH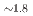 with H
with H fluid at pressures above 4 GPa and subsequent decompression to ambient pressure. It consists of complex SiH
fluid at pressures above 4 GPa and subsequent decompression to ambient pressure. It consists of complex SiH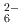 ions, which are octahedrally coordinated by Ba
ions, which are octahedrally coordinated by Ba counterions. The arrangement of Ba and Si atoms deviates only slightly from an ideal fcc NaCl structure. IR and Raman spectroscopy showed SiH
counterions. The arrangement of Ba and Si atoms deviates only slightly from an ideal fcc NaCl structure. IR and Raman spectroscopy showed SiH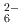 bending and stretching modes in the ranges 800-1200 and 1400-1800 cm
bending and stretching modes in the ranges 800-1200 and 1400-1800 cm , respectively. BaSiH
, respectively. BaSiH is thermally stable up to 95
is thermally stable up to 95 C above which decomposition into BaH
C above which decomposition into BaH and Si takes place. DFT calculations indicated a direct band gap of 2.5 eV. The discovery of BaSiH
and Si takes place. DFT calculations indicated a direct band gap of 2.5 eV. The discovery of BaSiH consolidates the compound class of hydridosilicates, accessible from hydrogenations of silicides at gigapascal pressures (
consolidates the compound class of hydridosilicates, accessible from hydrogenations of silicides at gigapascal pressures (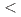 10 GPa). The structural properties of BaSiH
10 GPa). The structural properties of BaSiH suggest that it presents an intermediate (or precursor) for further hydrogenation at considerably higher pressures to the predicted superconducting polyhydride BaSiH
suggest that it presents an intermediate (or precursor) for further hydrogenation at considerably higher pressures to the predicted superconducting polyhydride BaSiH .
.
Niu, X.*; Elakneswaran, Y.*; Li, A.*; Seralathan, S.*; Kikuchi, Ryosuke*; Hiraki, Yoshihisa; Sato, Junya; Osugi, Takeshi; Walkley, B.*
Cement and Concrete Research, 190, p.107814_1 - 107814_17, 2025/04
Times Cited Count:0 Percentile:0.00(Construction & Building Technology)Collaborative Laboratories for Advanced Decommissioning Science; Ibaraki University*
JAEA-Review 2023-021, 112 Pages, 2024/02
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2018, this report summarizes the research results of the "Contribution to Risk Reduction in Decommissioning Works by the Elucidation of Basic Property of Radioactive Microparticles" conducted from FY2018 to FY2021 (this contract was extended to FY2021). The present study aims to understand the basic properties (size, chemical composition, isotopic composition - including concentration of  -emitters, electrostatic properties, and optical properties, etc.) of fine particles composed of silicate with insoluble properties which contain regions of highly concentrated radioactive cesium (Cs) released to the environment by the accident at the Fukushima Daiichi Nuclear Power Station of TEPCO in 2011 March.
-emitters, electrostatic properties, and optical properties, etc.) of fine particles composed of silicate with insoluble properties which contain regions of highly concentrated radioactive cesium (Cs) released to the environment by the accident at the Fukushima Daiichi Nuclear Power Station of TEPCO in 2011 March.
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 40(2), p.347 - 352, 2024/02
Times Cited Count:2 Percentile:11.65(Chemistry, Analytical)The Eu(III) distribution mechanism in single extractant-impregnated polymer-layered silica particle in a complex solution containing multiple lanthanide ions was investigated using fluorescence microspectroscopy. The rate-determining step was the reaction of Eu(III) with the two extractant molecules. The obtained mechanism and rate constants agreed with those of the single-ion distribution system, in which Eu(III) was distributed to the particles in the Eu(III) solution.
Kim, G.*; Cho, S.-M.*; Im, S.*; Suh, H.*; Morooka, Satoshi; Shobu, Takahisa; Kanematsu, Manabu*; Machida, Akihiko*; Bae, S.*
Construction and Building Materials, 411, p.134529_1 - 134529_18, 2024/01
Times Cited Count:8 Percentile:69.96(Construction & Building Technology)Cho, S.*; Suh, H.*; Im, S.*; Kim, G.*; Kanematsu, Manabu*; Morooka, Satoshi; Machida, Akihiko*; Shobu, Takahisa; Bae, S.*
Construction and Building Materials, 409, p.133866_1 - 133866_20, 2023/12
Times Cited Count:13 Percentile:85.36(Construction & Building Technology) -tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degree
-tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degreeMiyagawa, Akihisa*; Takahashi, Takumi*; Kuzure, Yoshiaki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 39(11), p.1929 - 1936, 2023/11
Times Cited Count:1 Percentile:11.65(Chemistry, Analytical)In this study, we revealed the Eu(III) distribution in a single diglycolamide-derivative extractant (TODGA)-impregnated polymer-coated silica particle. The reaction of Eu(III) with two TODGA molecules in the polymer layer was the rate-limiting process, as evidenced by the absence of any correlation between the rate constants (k and k
and k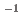 ) and concentrations of Eu(III) and HNO
) and concentrations of Eu(III) and HNO .
.
Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 96(9), p.1019 - 1025, 2023/09
Times Cited Count:4 Percentile:34.10(Chemistry, Multidisciplinary)In the present study, we have elucidated the mass transfer mechanism of Eu(III) and Sm(III) in the solution with these ions in single nitrilotriacetamide (NTA) extractant-impregnated polymer-coated silica particle. The rate-limiting process of mass transfer was the reaction process of ions with NTA molecules, in which the NO
 ions were not involved, which was consistent with that obtained in single ion distribution system.
ions were not involved, which was consistent with that obtained in single ion distribution system.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:7 Percentile:58.25(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
 synchrotron X-ray scattering and nanoindentation test
synchrotron X-ray scattering and nanoindentation testIm, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Choe, H.*; Nishio, Yuhei*; Machida, Akihiko*; Kim, J.*; Lim, S.*; et al.
Construction and Building Materials, 365, p.130034_1 - 130034_18, 2023/02
Times Cited Count:17 Percentile:77.01(Construction & Building Technology)Miyakawa, Kazuya; Kashiwaya, Koki*; Komura, Yuto*; Nakata, Kotaro*
Geochemical Journal, 57(5), p.155 - 175, 2023/00
Times Cited Count:2 Percentile:32.37(Geochemistry & Geophysics)In the thick marine sediments, groundwater altered from seawater during the burial diagenesis may exist. Such altered ancient seawater will be called fossil seawater. In such a field, groundwater flow is considered extremely slow because it is not affected by the seepage of meteoric water even after the uplift. During diagenesis, dehydration from silicates causes changes such as a decrease in the salinity of the porewater. However, dehydration reactions alone cannot quantitatively explain water chemistry changes. In this study, we developed an analytical model that considers the dehydration reaction from silicates during the burial process and the upward migration of porewater due to compaction and examined the possible evolution of porewater chemistry. The results showed that the water chemistry, which was strongly influenced by the dehydration reaction from opal-A to quartz and from smectite, was similar to the observations from boring surveys. The results suggest that the fossil seawater formed during the diagenesis may have been preserved since the uplift and strongly supports the slow groundwater flow in the area where the fossil seawater exists.
Kim, G.*; Im, S.*; Jee, H.*; Suh, H.*; Cho, S.*; Kanematsu, Manabu*; Morooka, Satoshi; Koyama, Taku*; Nishio, Yuhei*; Machida, Akihiko*; et al.
Cement and Concrete Research, 159, p.106869_1 - 106869_17, 2022/09
Times Cited Count:27 Percentile:86.85(Construction & Building Technology) and GeO
and GeO liquids
liquidsKawaguchi, Munemichi; Uno, Masayoshi*
Journal of Crystal Growth, 585, p.126590_1 - 126590_7, 2022/05
Phase-field mobility,  , and crystal growth rates in crystallization of 11 oxides or mixed oxides in undercooled silicates, SiO
, and crystal growth rates in crystallization of 11 oxides or mixed oxides in undercooled silicates, SiO and GeO
and GeO liquids were calculated with a simple phase-field model (PFM), and material dependence of the
liquids were calculated with a simple phase-field model (PFM), and material dependence of the  was discussed. Ratios between experimental crystal growth rates and the PFM simulation with
was discussed. Ratios between experimental crystal growth rates and the PFM simulation with 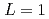 were confirmed to be proportional to a power of
were confirmed to be proportional to a power of 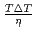 on the solid/liquid interface process during the crystal growth in a log-log plot. We determined that parameters,
on the solid/liquid interface process during the crystal growth in a log-log plot. We determined that parameters, 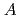 and
and  , of the
, of the 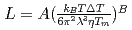 were
were 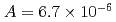 to
to 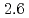 m
m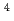 J
J s
s and
and 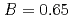 to
to 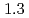 , which were unique for the materials. It was confirmed that our PFM simulation with the determined
, which were unique for the materials. It was confirmed that our PFM simulation with the determined  reproduced quantitively the experimental crystal growth rates. The
reproduced quantitively the experimental crystal growth rates. The 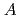 has a proportional relationship with the diffusion coefficient of a cation molar mass average per unit an oxygen molar mass at
has a proportional relationship with the diffusion coefficient of a cation molar mass average per unit an oxygen molar mass at 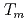 in a log-log graph. The
in a log-log graph. The  depends on the sum of the cation molar mass per the oxygen molar mass,
depends on the sum of the cation molar mass per the oxygen molar mass, 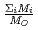 , in a compound. In
, in a compound. In 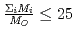 , the
, the  decreases with the cation molar mass increasing. The assumed cause is that the B represents the degree of the temperature dependence of the
decreases with the cation molar mass increasing. The assumed cause is that the B represents the degree of the temperature dependence of the  . Since the cation molar mass is proportional to an inertial resistance of the cation transfer, the
. Since the cation molar mass is proportional to an inertial resistance of the cation transfer, the  decreases with inverse of the cation molar mass. In crystallization of the silicates of heavy cation in
decreases with inverse of the cation molar mass. In crystallization of the silicates of heavy cation in 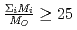 , the
, the  saturates at approximately 0.67, which leads to
saturates at approximately 0.67, which leads to 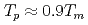 .
.
Im, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Koyama, Taku*; Nishio, Yuhei*; Machida, Akihiko*; Kim, J.*; Bae, S.*
Journal of the American Ceramic Society, 104(9), p.4803 - 4818, 2021/09
Times Cited Count:26 Percentile:82.77(Materials Science, Ceramics)Myagmarjav, O.; Tanaka, Nobuyuki; Nomura, Mikihiro*; Noguchi, Hiroki; Imai, Yoshiyuki; Kamiji, Yu; Kubo, Shinji; Takegami, Hiroaki
Progress in Nuclear Energy, 137, p.103772_1 - 103772_7, 2021/07
Times Cited Count:8 Percentile:67.14(Nuclear Science & Technology)Sato, Junya; Shiota, Kenji*; Takaoka, Masaki*
Zairyo, 70(5), p.406 - 411, 2021/05
An aluminosilicate solid is an inorganic material that has the property of immobilizing heavy metals or radionuclides in the matrix. In this study, aluminosilicates with a Si/Al molar ratio of 0.5 was synthesized from a chemical reagent in order to produce aluminosilicate solids with a low Si/Al molar ratio, which were expected to improve the immobilization of heavy metals and radionuclides contained in the matrix. The synthesized Si-Al gel with a Si/Al molar ratio of 0.5 had little impurity content and was in an amorphous phase. In addition, the compressive strength of the aluminosilicate solid produced by the synthesized Si-Al gel showed a 5 MPa or more, confirming that it can be used as a raw material for aluminosilicate solids. The aluminosilicate solid with a Si/Al molar ratio of 1.25 had a dense surface structure from the result of BSE images and had the highest compressive strength among all samples.
Collaborative Laboratories for Advanced Decommissioning Science; Ibaraki University*
JAEA-Review 2020-033, 84 Pages, 2021/01
JAEA/CLADS had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project in FY2019. Among the adopted proposals in FY2018, this report summarizes the research results of the "Contribution to Risk Reduction in Decommissioning Works by the Elucidation of Basic Property of Radioactive Microparticles" conducted in FY2019.