Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:4 Percentile:51.78(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U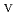
 Fe
Fe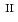
 U
U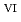 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Rizaal, M.; Nakajima, Kunihisa; Saito, Takumi*; Osaka, Masahiko; Okamoto, Koji*
Journal of Nuclear Science and Technology, 57(9), p.1062 - 1073, 2020/09
Times Cited Count:9 Percentile:64.46(Nuclear Science & Technology)The interaction of cesium hydroxide and a calcium silicate insulation material was experimentally investigated at high temperature conditions. A thermogravimetry equipped with differential thermal analysis was used to analyze thermal events in the samples of mixed calcium silicate and cesium hydroxide under Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 with maximum temperature of 1100
0 with maximum temperature of 1100 C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca
C. Prior being mixed with cesium hydroxide, a part of calcium silicate was pretreated at high temperature to evaluate the effect of possible structural changes of this material due to a preceding thermal history and also the sake of thermodynamic evaluation to those available ones. Based upon the initial condition (preliminary heat treatment) of calcium silicate, it was found that if the original material consisted of xonotlite (Ca Si
Si 0
0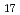 (0H)
(0H) ), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730
), the endothermic reaction with cesium hydroxide occurred over the temperature range 575-730 C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0
C meanwhile if the crystal phase of original material was changed to wollastonite (CaSi0 ), the interaction occurred over temperature range 700-1100
), the interaction occurred over temperature range 700-1100 C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H
C. Furthermore, the X-ray diffraction analyses have indicated on both type of pretreated calsils that regardless of Ar-5%H and Ar-4%H
and Ar-4%H -20%H
-20%H 0 atmosphere, cesium aluminum silicate, CsAlSi0
0 atmosphere, cesium aluminum silicate, CsAlSi0 was formed with aluminum in the samples as an impurity or adduct.
was formed with aluminum in the samples as an impurity or adduct.
 U captures to
U captures to  U fission in low-enriched UO
U fission in low-enriched UO tight lattices
tight latticesNakajima, Ken; ; Yamamoto, Toshihiro; ; Suzaki, Takenori
Journal of Nuclear Science and Technology, 31(11), p.1160 - 1170, 1994/11
Times Cited Count:7 Percentile:56.04(Nuclear Science & Technology)no abstracts in English
 cores
coresNakajima, Ken; ; Suzaki, Takenori
Nuclear Science and Engineering, 116, p.138 - 146, 1994/00
Times Cited Count:16 Percentile:78.03(Nuclear Science & Technology)no abstracts in English
Masukawa, Fumihiro; ; Inoue, Osamu*; Hara, Toshiharu*
JAERI-M 93-024, 31 Pages, 1993/02
no abstracts in English
Nakano, Yoshihiro
JAERI-M 92-103, 67 Pages, 1992/07
no abstracts in English
 -UO
-UO fuel assembly
fuel assemblyC-S.Gil*; Okumura, Keisuke;
JAERI-M 91-200, 61 Pages, 1991/11
no abstracts in English
Okuno, Hiroshi; Naito, Yoshitaka; Sakurai, Y.*
Journal of Nuclear Science and Technology, 28(10), p.958 - 960, 1991/10
no abstracts in English
Okumura, Keisuke; ; Tanaka, Kenichi*; C-J.Jeong*
JAERI-M 89-201, 150 Pages, 1989/12
no abstracts in English
; ;
Journal of Nuclear Science and Technology, 24(8), p.610 - 620, 1987/08
Times Cited Count:4 Percentile:44.56(Nuclear Science & Technology)no abstracts in English
; ;
JAERI-M 86-190, 52 Pages, 1987/01
no abstracts in English
Nakajima, Hayato; Shimizu, Saburo; ; Ikezoe, Yasumasa;
Nihon Kagakkai-Shi, 8, p.1257 - 1261, 1984/00
no abstracts in English


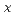 (0≦x≦0.1)
(0≦x≦0.1)Ugajin, Mitsuhiro
Journal of Nuclear Science and Technology, 20(3), p.228 - 236, 1983/00
Times Cited Count:20 Percentile:85.98(Nuclear Science & Technology)no abstracts in English
;
Radiochem.Radioanal.Lett., 29(6), p.331 - 340, 1977/06
no abstracts in English