Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 neutron diffraction study
neutron diffraction studyYamashita, Takayuki*; Koga, Norimitsu*; Mao, W.*; Gong, W.; Kawasaki, Takuro; Harjo, S.; Fujii, Hidetoshi*; Umezawa, Osamu*
Materials Science and Engineering A, 941, p.148602_1 - 148602_11, 2025/09
Times Cited Count:0Gu, G. H.*; Jeong, S. G.*; Heo, Y.-U.*; Harjo, S.; Gong, W.; Cho, J.*; Kim, H. S.*; 4 of others*
Journal of Materials Science & Technology, 223, p.308 - 324, 2025/07
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Mao, W.*; Gong, W.; Kawasaki, Takuro; Gao, S.*; Ito, Tatsuya; Yamashita, Takayuki*; Harjo, S.; Zhao, L.*; Wang, Q.*
Scripta Materialia, 264, p.116726_1 - 116726_6, 2025/07
Times Cited Count:0 Percentile:0.00(Nanoscience & Nanotechnology)Park, M.-H.*; Shibata, Akinobu*; Harjo, S.; Tsuji, Nobuhiro*
Acta Materialia, 292, p.121061_1 - 121061_13, 2025/06
Times Cited Count:1 Percentile:84.88(Materials Science, Multidisciplinary)Ohashi, Tomonori*; Sakamaki, Tatsuya*; Funakoshi, Kenichi*; Steinle-Neumann, G.*; Hattori, Takanori; Yuan, L.*; Suzuki, Akio*
Journal of Mineralogical and Petrological Sciences (Internet), 120(1), p.240926a_1 - 240926a_13, 2025/06
We explore the structures of dry and hydrated (H O and D
O and D O) Na
O) Na Si
Si O
O melt at 0-6 GPa and 1000-1300 K and glasses recovered from high pressure and temperatures by in-situ neutron and X-ray diffraction. The structures of the melts at 0-10 GPa and 3000 K are also investigated by ab-initio molecular dynamics simulation. In-situ neutron experiments revealed that the D-O distance increases with compression due to the formation of -O-D-O- bridging species, which is reproduced by the molecular dynamics simulations. The pressure-induced -O-D-O- formation reflects a more rigid incorporation of hydrogen, which acts as a mechanism for the experimentally observed higher solubility of water in silicate melts. Together with shrinking modifier domains, this process dominates the compression behavior of hydrous Na
melt at 0-6 GPa and 1000-1300 K and glasses recovered from high pressure and temperatures by in-situ neutron and X-ray diffraction. The structures of the melts at 0-10 GPa and 3000 K are also investigated by ab-initio molecular dynamics simulation. In-situ neutron experiments revealed that the D-O distance increases with compression due to the formation of -O-D-O- bridging species, which is reproduced by the molecular dynamics simulations. The pressure-induced -O-D-O- formation reflects a more rigid incorporation of hydrogen, which acts as a mechanism for the experimentally observed higher solubility of water in silicate melts. Together with shrinking modifier domains, this process dominates the compression behavior of hydrous Na Si
Si O
O melt, whereas the compression of dry Na
melt, whereas the compression of dry Na Si
Si O
O at 0-10 GPa and 3000 K is governed largely by bending of the Si-O-Si angle. The molecular dynamics simulations on hydrous Na
at 0-10 GPa and 3000 K is governed largely by bending of the Si-O-Si angle. The molecular dynamics simulations on hydrous Na Si
Si O
O melts further suggest that the sodium ions are scavenged from its network-modifying role via 2(
melts further suggest that the sodium ions are scavenged from its network-modifying role via 2(![$$^{[4]}$$](/search/images/ERPHWWZULV4SI.gif) Si-O
Si-O + Na
+ Na )
) 
![$$^{[4]}$$](/search/images/ERPHWWZULV4SI.gif) Si-(O-
Si-(O-![$$^{[5]}$$](/search/images/ERPHWWZVLV4SI.gif) Si-O)
Si-O) + 2Na
+ 2Na and Si-O
and Si-O + Na
+ Na + Si-OH
+ Si-OH  Si-(O-H-O-Si)
Si-(O-H-O-Si) + Na
+ Na with increasing pressure.
with increasing pressure.
Tomota, Yo*; Harjo, S.; Xu, P. G.; Morooka, Satoshi; Gong, W.; Wang, Y.*
Metals, 15(6), p.610_1 - 610_19, 2025/05
Times Cited Count:0 phases in undoped and Ca-modified sodium niobates
phases in undoped and Ca-modified sodium niobatesAso, Seiyu*; Matsuo, Hiroki*; Yoneda, Yasuhiro; Morikawa, Daisuke*; Tsuda, Kenji*; Oyama, Kenji*; Ishigaki, Toru*; Noguchi, Yuji*
Physical Review B, 111(17), p.174114_1 - 174114_12, 2025/05
We investigate the crystal structures, phase transitions, and phase stability of undoped and Ca-modified NaNbO through a combined analysis of high-resolution synchrotron radiation X-ray and neutron diffraction, convergent-beam electron diffraction, and density functional theory (DFT) calculations. It is demonstrated that the antiferroelectric (AFE)-
through a combined analysis of high-resolution synchrotron radiation X-ray and neutron diffraction, convergent-beam electron diffraction, and density functional theory (DFT) calculations. It is demonstrated that the antiferroelectric (AFE)- phase is stabilized over a wide temperature range of 200 to 800 K by Ca modification, and that the NaNbO
phase is stabilized over a wide temperature range of 200 to 800 K by Ca modification, and that the NaNbO is stabilized by temperature-driven isostatic pressure accompanied by lattice expansion, whereas the Ca-modified NaNbO
is stabilized by temperature-driven isostatic pressure accompanied by lattice expansion, whereas the Ca-modified NaNbO is induced by composition-induced chemical pressure along with lattice shrinkage.
is induced by composition-induced chemical pressure along with lattice shrinkage.
 neutron diffraction study
neutron diffraction studyIto, Tatsuya; Ogawa, Yuhei*; Gong, W.; Mao, W.*; Kawasaki, Takuro; Okada, Kazuho*; Shibata, Akinobu*; Harjo, S.
Acta Materialia, 287, p.120767_1 - 120767_16, 2025/04
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Naeem, M.*; Rehman, A. U.*; Romero Resendiz, L.*; Salamci, E.*; Aydin, H.*; Ansari, P.*; Harjo, S.; Gong, W.; Wang, X.-L.*; 3 of others*
Communications Materials (Internet), 6, p.65_1 - 65_13, 2025/04
Pandian, K.*; Neikter, M.*; Ekh, M.*; Harjo, S.; Kawasaki, Takuro; Woracek, R.*; Hansson, T.*; Pederson, R.*
JOM, 77(4), p.1803 - 1815, 2025/04
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary) under hydrostatic pressure; A Combined neutron, X-ray, Raman, and first-principles calculation study
under hydrostatic pressure; A Combined neutron, X-ray, Raman, and first-principles calculation studyEfthimiopoulos, I.*; Klotz, S.*; Kunc, K.*; Baptiste, B.*; Chauvigne, P.*; Hattori, Takanori
Physical Review B, 111(13), p.134103_1 - 134103_13, 2025/04
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)We present a comprehensive study of the high pressure behaviour of ReO using X-ray and neutron diffraction, Raman scattering and first-principles calculations to 15 GPa. We show that the ambient pressure
using X-ray and neutron diffraction, Raman scattering and first-principles calculations to 15 GPa. We show that the ambient pressure 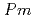
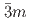 structure converts at 0.7 GPa in a continuous phase transition directly to a cubic phase with space group
structure converts at 0.7 GPa in a continuous phase transition directly to a cubic phase with space group 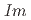
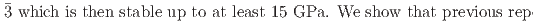 and rhombohedral
and rhombohedral 
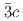 structures in this pressure range are an artifact due to an alteration of the sample by high-flux synchrotron X-ray radiation. The structural pressure dependence of the
structures in this pressure range are an artifact due to an alteration of the sample by high-flux synchrotron X-ray radiation. The structural pressure dependence of the 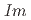
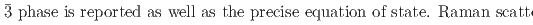 O samples are presented. The data shed light onto the unusual transition and densification mechanism due to progressive tilting of essentially rigid ReO
O samples are presented. The data shed light onto the unusual transition and densification mechanism due to progressive tilting of essentially rigid ReO octahedra.
octahedra.
 GeTe
GeTe to hydrostatic pressure
to hydrostatic pressureWang, Y.*; Zeng, X.-T.*; Li, B.*; Su, C.*; Hattori, Takanori; Sheng, X.-L.*; Jin, W.*
Chinese Physics B, 34(4), p.046203_1 - 046203_6, 2025/03
Times Cited Count:0 Percentile:0.00(Physics, Multidisciplinary)Two-dimensional van der Waals ferromagnet Fe GeTe
GeTe (FGT) holds a great potential for applications in spintronic devices, due to its high Curie temperature, easy tunability, and excellent structural stability in air. In this study, we have performed high-pressure neutron powder diffraction (NPD) up to 5 GPa, to investigate the evolution of its structural and magnetic properties with hydrostatic pressure. The NPD data clearly reveal the robustness of the ferromagnetism in FGT, despite of an apparent suppression by hydrostatic pressure. As the pressure increases from 0 to 5 GPa, the Curie temperature is found to decrease monotonically from 225(5) K to 175(5) K, together with a dramatically suppressed ordered moment of Fe, which is well supported by the first-principles calculations. Although no pressure-driven structural phase transition is observed up to 5 GPa, quantitative analysis on the changes of bond lengths and bond angles indicate a significant modification of the exchange interactions, which accounts for the pressure-induced suppression of the ferromagnetism in FGT.
(FGT) holds a great potential for applications in spintronic devices, due to its high Curie temperature, easy tunability, and excellent structural stability in air. In this study, we have performed high-pressure neutron powder diffraction (NPD) up to 5 GPa, to investigate the evolution of its structural and magnetic properties with hydrostatic pressure. The NPD data clearly reveal the robustness of the ferromagnetism in FGT, despite of an apparent suppression by hydrostatic pressure. As the pressure increases from 0 to 5 GPa, the Curie temperature is found to decrease monotonically from 225(5) K to 175(5) K, together with a dramatically suppressed ordered moment of Fe, which is well supported by the first-principles calculations. Although no pressure-driven structural phase transition is observed up to 5 GPa, quantitative analysis on the changes of bond lengths and bond angles indicate a significant modification of the exchange interactions, which accounts for the pressure-induced suppression of the ferromagnetism in FGT.
Aoki, Katsutoshi*; Machida, Akihiko*; Saito, Hiroyuki*; Hattori, Takanori
Koatsuryoku No Kagaku To Gijutsu, 35(1), p.4 - 11, 2025/03
Iron reacts with hydrogen to form solid solutions with body-centered cubic, face-centered cubic, hexagonal close packed, and double hexagonal close packed structures at high temperatures and high pressures. Neutron diffraction is the most powerful tool for determining the occupation sites and occupancies of hydrogen atoms dissolved in a metal lattice. Structural parameters, including hydrogen occupation sites and occupancies, are refined via Rietveld analysis for neutron diffraction data. We present our expertise in Rietveld refinement of iron hydrides accumulated over 10 years.
Xu, J.*; Lang, P.*; Liang, S.*; Zhang, J.*; Fei, Y.*; Wang, Y.*; Gao, D.*; Hattori, Takanori; Abe, Jun*; Dong, X.*; et al.
Journal of Physical Chemistry Letters (Internet), p.2445 - 2451, 2025/00
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)The Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen, and it provides an efficient method to construct the C-C bond. Traditionally, this reaction requires catalysts, high temperatures, or photocatalysis. In this study, we reported a high-pressure-induced solid-state Alder-ene reaction of 1-hexene at room temperature without a catalyst. 1-Hexene crystallizes at 4.3 GPa and polymerizes at 18 GPa, forming olefins. By exploring gas chromatography-mass spectrometry, we discovered that 1-hexene generates dimeric products through the Alder-ene reaction under high pressures. The in situ neutron diffraction shows that the reaction process did not obey the topochemical rule. A six-membered ring transition state including one C-H  bond and two alkene
bond and two alkene  bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
 neutron diffraction measurement during tensile deformation
neutron diffraction measurement during tensile deformationYamashita, Takayuki*; Morooka, Satoshi; Gong, W.; Kawasaki, Takuro; Harjo, S.; Hojo, Tomohiko*; Okitsu, Yoshitaka*; Fujii, Hidetoshi*
ISIJ International, 64(14), p.2051 - 2060, 2024/12
Times Cited Count:0Materials Sciences Research Center
JAEA-Review 2024-037, 141 Pages, 2024/11
Fifteen neutron beam experimental instruments managed by JAEA are installed in JRR-3 (Japan Research Reactor No.3) and are available for internal use including upgrading of instruments and for external users to produce various research results. This report summarizes the progress of internal application research and technical development such as upgrading of neutron beam instruments in the fiscal years 2021 and 2022 after the restart of operation.
Harjo, S.; Mao, W.*; Gong, W.; Kawasaki, Takuro
Proceedings of the 7th International Symposium on Steel Science (ISSS 2024), p.205 - 208, 2024/11
 neutron diffraction study to elucidate hydrogen effect on the deformation mechanism in Type 310S austenitic stainless steel
neutron diffraction study to elucidate hydrogen effect on the deformation mechanism in Type 310S austenitic stainless steelIto, Tatsuya; Ogawa, Yuhei*; Gong, W.; Mao, W.*; Kawasaki, Takuro; Okada, Kazuho*; Shibata, Akinobu*; Harjo, S.
Proceedings of the 7th International Symposium on Steel Science (ISSS 2024), p.237 - 240, 2024/11
Mao, W.*; Gao, S.*; Gong, W.; Kawasaki, Takuro; Ito, Tatsuya; Harjo, S.; Tsuji, Nobuhiro*
Acta Materialia, 278, p.120233_1 - 120233_13, 2024/10
Times Cited Count:13 Percentile:96.74(Materials Science, Multidisciplinary)Zhang, Y.-J.*; Umeda, Takemasa*; Morooka, Satoshi; Harjo, S.; Miyamoto, Goro*; Furuhara, Tadashi*
Metallurgical and Materials Transactions A, 55(10), p.3921 - 3936, 2024/10
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)