Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fujihara, Masayoshi*; Morita, Katsuhiro*; Mole, R.*; Mitsuda, Setsuo*; Toyama, Takami*; Yano, Shinichiro*; Yu, D.*; Sota, Shigetoshi*; Kuwai, Tomohiko*; Koda, Akihiro*; et al.
Nature Communications (Internet), 11, p.3429_1 - 3429_7, 2020/07
Times Cited Count:39 Percentile:92.14(Multidisciplinary Sciences)Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:72.98(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
 and W(CO)
and W(CO)
Usoltsev, I.*; Eichler, R.*; Wang, Y.*; Even, J.*; Yakushev, A.*; Haba, Hiromitsu*; Asai, Masato; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; et al.
llmann, Ch. E.*; et al.
Radiochimica Acta, 104(3), p.141 - 151, 2016/03
Times Cited Count:31 Percentile:94.91(Chemistry, Inorganic & Nuclear)Conditions of the production and decomposition of hexacarbonyl complexes of short-lived Mo and W isotopes were investigated to study thermal stability of the heaviest group 6 hexacarbonyl complex Sg(CO) . A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO)
. A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO) and W(CO)
and W(CO) , indicating that the first bond dissociation energy of Sg(CO)
, indicating that the first bond dissociation energy of Sg(CO) could be determined with this technique.
could be determined with this technique.
Even, J.*; Ackermann, D.*; Asai, Masato; Block, M.*; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(3), p.2457 - 2466, 2015/03
Times Cited Count:15 Percentile:77.35(Chemistry, Analytical)Rapid In situ synthesis of metal carbonyl complexes has been demonstrated using short-lived isotopes produced in nuclear fission and fusion reactions. The short-lived isotopes with high recoil energy directly react with carbon-monoxides and form carbonyl complexes. Only highly volatile complexes were fast transported in a gas stream to counting and chemistry devices. Short-lived Mo, Tc, Ru, Rh, W, Re, Os, and Ir were found to form volatile carbonyl complexes, while no volataile complex of Hf and Ta were detected. This technique has been applied to a chemical investigation of the superheavy element Sg (atomic number 106), and will be applicable to various fields of nuclear science with short-lived transition metal isotopes.
Even, J.*; Yakushev, A.*; D llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
Science, 345(6203), p.1491 - 1493, 2014/09
Times Cited Count:64 Percentile:83.15(Multidisciplinary Sciences)A new superheavy element complex, a seaborgium carbonyl, has been successfully synthesized, and its adsorption property has been studied using a cryo-thermochromatography and  -detection apparatus COMPACT. Nuclear reaction products of short-lived
-detection apparatus COMPACT. Nuclear reaction products of short-lived  Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of
Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of 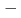 50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO)
50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO) . This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
. This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
Uosaki, Kohei*; Morita, Jun*; Katsuzaki, Tomoko*; Takakusagi, Satoru*; Tamura, Kazuhisa; Takahashi, Masamitsu; Mizuki, Junichiro; Kondo, Toshihiro*
Journal of Physical Chemistry C, 115(25), p.12471 - 12482, 2011/06
Times Cited Count:13 Percentile:40.03(Chemistry, Physical)Ag/AgCl reaction at the Ag bilayer, which was underpotentially prepared on a Au(111) surface, was investigated using electrochemical quartz crystal microbalance (EQCM), scanning tunneling microscopy (STM), surface X-ray scattering (SXS), and electrochemical techniques. When the potential was scanned positively from -200 mV, the Cl ion was adsorbed on the Au(111) electrode surface around 0 mV, and then the phase transition of the adsorbed Cl
ion was adsorbed on the Au(111) electrode surface around 0 mV, and then the phase transition of the adsorbed Cl ion layer from random orientation to (
ion layer from random orientation to (

 ) structure took place at around +130 mV. The Ag bilayer and Cl
) structure took place at around +130 mV. The Ag bilayer and Cl ions were oxidatively reacted to form the AgCl monolayer with (
ions were oxidatively reacted to form the AgCl monolayer with (

 )
)  13.9
13.9 structure around +200 mV, accompanied with the formation of AgCl monocrystalline clusters on the AgCl monolayer surface. The structure of the AgCl monolayer on the Au(111) surface was changed from (
structure around +200 mV, accompanied with the formation of AgCl monocrystalline clusters on the AgCl monolayer surface. The structure of the AgCl monolayer on the Au(111) surface was changed from (

 )R13.9
)R13.9 structure to (4
structure to (4 4) structure around +500 mV.
4) structure around +500 mV.
Morita, Takami*; Niwa, Kentaro*; Fujimoto, Ken*; Kasai, Hiromi*; Yamada, Haruya*; Nishiuchi, Ko*; Sakamoto, Tatsuya*; Godo, Waichiro*; Taino, Seiya*; Hayashi, Yoshihiro*; et al.
Science of the Total Environment, 408(16), p.3443 - 3447, 2010/06
Times Cited Count:13 Percentile:32.9(Environmental Sciences)Iodine-131 ( I) was detected in brown algae collected off the Japanese coast. The maximum measured specific activity of
I) was detected in brown algae collected off the Japanese coast. The maximum measured specific activity of  I in brown algae was 0.37
I in brown algae was 0.37 0.010 Bq/kg-wet. Cesium-137 (
0.010 Bq/kg-wet. Cesium-137 ( Cs) was also detected in all brown algal samples used in this study. There was no correlation between specific activities of
Cs) was also detected in all brown algal samples used in this study. There was no correlation between specific activities of  I and
I and  Cs in these seaweeds. Low specific activity and minimal variability of
Cs in these seaweeds. Low specific activity and minimal variability of  Cs in brown algae indicated that past nuclear weapon tests were the source of
Cs in brown algae indicated that past nuclear weapon tests were the source of  Cs. Although nuclear power facilities are known to be pollution sources of
Cs. Although nuclear power facilities are known to be pollution sources of  I, there was no relationship between the sites where
I, there was no relationship between the sites where  I was detected and the locations of nuclear power facilities. Most of the sites where
I was detected and the locations of nuclear power facilities. Most of the sites where  I was detected were near big cities with large populations. On the basis of the results, we suggest that the likely pollution source of
I was detected were near big cities with large populations. On the basis of the results, we suggest that the likely pollution source of  I, detected in brown seaweeds, is not nuclear power facilities, but nuclear medicine procedures.
I, detected in brown seaweeds, is not nuclear power facilities, but nuclear medicine procedures.
Kumamoto, Yuichiro*; Aramaki, Takafumi*; Watanabe, Shuichi*; Yoneda, Minoru*; Shibata, Yasuyuki*; Togawa, Orihiko; Morita, Masatoshi*; Shitashima, Kiminori*
Journal of Oceanography, 64(3), p.429 - 441, 2008/06
Times Cited Count:11 Percentile:25.29(Oceanography)In 1995 and 2000, radiocarbon ratio (
 C) of total dissolved inorganic carbon was measured in the Japan Sea, a semi-closed marginal sea in the western North Pacific, where deep and bottom waters are formed in itself. Compiling them with historical radiocarbon data in the Japan Sea, temporal and spatial variations of the radiocarbon in the bottom water below 2000 m depth were elucidated.
C) of total dissolved inorganic carbon was measured in the Japan Sea, a semi-closed marginal sea in the western North Pacific, where deep and bottom waters are formed in itself. Compiling them with historical radiocarbon data in the Japan Sea, temporal and spatial variations of the radiocarbon in the bottom water below 2000 m depth were elucidated. 
 C in the bottom waters in the western Japan and Yamato Basins increased by about 20 ‰ between 1977/79 and 1995 and did not changed between 1995 and 1999/2000, suggesting penetration of surface bomb-produced radiocarbon into the bottom waters due to bottom ventilation in the earlier period and stagnation of the bottom ventilation in the following period, respectively. In the eastern Japan Basin, the bottom
C in the bottom waters in the western Japan and Yamato Basins increased by about 20 ‰ between 1977/79 and 1995 and did not changed between 1995 and 1999/2000, suggesting penetration of surface bomb-produced radiocarbon into the bottom waters due to bottom ventilation in the earlier period and stagnation of the bottom ventilation in the following period, respectively. In the eastern Japan Basin, the bottom 
 C increased by about 10 ‰ between 1977/79 and 2002, suggesting less ventilation of the bottom water in the basin. The temporal changes of the radiocarbon, tritium, and dissolved oxygen suggest sporadic occurrences of the bottom ventilation between 1979 and 1984 and its stagnation between 1984 and 2004 in the eastern Japan and Yamato Basins. The former is probably due to spreading of a newly ventilated bottom water in the western Japan Basin in the severe winter of 1976-1977 along the abyssal circulation in the Japan Sea. The latter does not conflict with temporal changes of bomb-produced
C increased by about 10 ‰ between 1977/79 and 2002, suggesting less ventilation of the bottom water in the basin. The temporal changes of the radiocarbon, tritium, and dissolved oxygen suggest sporadic occurrences of the bottom ventilation between 1979 and 1984 and its stagnation between 1984 and 2004 in the eastern Japan and Yamato Basins. The former is probably due to spreading of a newly ventilated bottom water in the western Japan Basin in the severe winter of 1976-1977 along the abyssal circulation in the Japan Sea. The latter does not conflict with temporal changes of bomb-produced  Cs and chlorofluorocarbon-11 in the bottom water.
Cs and chlorofluorocarbon-11 in the bottom water.
 -rays; Guineagrass and sorghum
-rays; Guineagrass and sorghumNakagawa, Hitoshi*; Inafuku, Masashi*; Kusaba, Makoto*; Yamaguchi, Hiroyasu*; Morishita, Toshikazu*; Morita, Ryohei*; Nishimura, Minoru*; Hoeman, S.*; Yokota, Yuichiro; Hase, Yoshihiro; et al.
JAEA-Review 2007-060, JAEA Takasaki Annual Report 2006, P. 72, 2008/03
no abstracts in English
Kondo, Toshihiro*; Morita, Jun*; Hanaoka, Kazuya*; Takakusagi, Satoru*; Tamura, Kazuhisa; Takahashi, Masamitsu; Mizuki, Junichiro; Uosaki, Kohei*
Journal of Physical Chemistry C, 111(35), p.13197 - 13204, 2007/09
Times Cited Count:84 Percentile:89.3(Chemistry, Physical)Potential-dependent surface structures of Au(111) and Au(100) single-crystal electrodes in a 50 mM H SO
SO solution were investigated at an atomic level using in situ surface X-ray scattering (SXS) techniques. It was confirmed that both the Au(111) and Au(100) surfaces were reconstructed with an attached submonolayer of an oxygen species, most probably water, at 0 V (vs Ag/AgCl). Results at +0.95 V supported a previously suggested model for both the Au(111) and the Au(100) electrodes that, based on infrared and scanning tunneling microscopy measurements, the surfaces were a (1
solution were investigated at an atomic level using in situ surface X-ray scattering (SXS) techniques. It was confirmed that both the Au(111) and Au(100) surfaces were reconstructed with an attached submonolayer of an oxygen species, most probably water, at 0 V (vs Ag/AgCl). Results at +0.95 V supported a previously suggested model for both the Au(111) and the Au(100) electrodes that, based on infrared and scanning tunneling microscopy measurements, the surfaces were a (1 1) structure with the coadsorbed sulfate anion and hydronium cation (H
1) structure with the coadsorbed sulfate anion and hydronium cation (H O
O ). At +1.05 V, where a small amount of an anodic current flowed, adsorption of a monolayer of oxygen species was observed on both surfaces.
). At +1.05 V, where a small amount of an anodic current flowed, adsorption of a monolayer of oxygen species was observed on both surfaces.
 ray
rayMorita, Ryohei*; Morishita, Toshikazu*; Nakagawa, Hitoshi*; Nishimura, Minoru*; Yamaguchi, Hiroyasu*; Yokota, Yuichiro; Hase, Yoshihiro; Tanaka, Atsushi
JAEA-Review 2006-042, JAEA Takasaki Annual Report 2005, P. 78, 2007/02
no abstracts in English
Morita, Yosuke; Yagi, Toshiaki; *
Denki Gakkai Yuden, Zetsuen Zairyo Kenkyukai Shiryo; DEI-99-12, p.23 - 26, 1999/02
no abstracts in English
Morita, Yosuke; Yagi, Toshiaki; *
Denki Gakkai Yuden, Zetsuen Zairyo Kenkyukai Shiryo; DEI-99-13, p.27 - 30, 1999/02
no abstracts in English
Oka, Kiyoshi; Obara, Kenjiro; Kakudate, Satoshi; *; *; Morita, Yosuke
Purazuma, Kaku Yugo Gakkai-Shi, 73(1), p.69 - 82, 1997/01
no abstracts in English
Ogiwara, Norio; *; Saido, Masahiro; *; Saito, Yoshio*; *; *; *; *
Journal of Nuclear Materials, 220-222, p.748 - 751, 1995/00
Times Cited Count:3 Percentile:36.71(Materials Science, Multidisciplinary)no abstracts in English
Yagi, Toshiaki; Morita, Yosuke; Seguchi, Tadao; *
Denki Gakkai Yuden, Zetsuen Zairyo Kenkyukai Shiryo; DEI-94-90, 0, p.21 - 28, 1994/12
no abstracts in English
 O,O
O,O and energetic O
and energetic O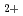 on boron carbide
on boron carbideOgiwara, Norio; *; Saido, Masahiro; *; Saito, Yoshio*; *; *
Journal of Nuclear Materials, 212-215, p.1260 - 1265, 1994/00
Times Cited Count:7 Percentile:56.56(Materials Science, Multidisciplinary)no abstracts in English
Obara, Kenjiro; Kakudate, Satoshi; Tada, Eisuke; Morita, Yosuke; *
Shinku, 37(3), p.124 - 127, 1994/00
no abstracts in English
*; Kamimura, Katsuichiro; Kodato, Kazuo; Yamaguchi, Toshihiro; *
PNC TN841 84-23, 190 Pages, 1984/05
no abstracts in English
Kambara, Toyozo; Uno, Hidero; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Kohayakawa, Toru; Takayanagi, Hiroshi; Fujimura, Tsutomu; Morita, Morito; Ichihara, Masahiro; et al.
JAERI 1045, 11 Pages, 1963/03
no abstracts in English