Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Morishita, Kazuki; Sato, Takumi; Onishi, Takashi; Seki, Takayuki*; Sekine, Shinichi*; Okitsu, Yuichi*
JAEA-Technology 2021-024, 27 Pages, 2021/10
In the case of Plutonium (Pu)-bearing organic materials, organic materials are decomposed by alpha rays emitted mainly from Pu to generate hydrogen gas and other substances. Therefore, to safely store Pu-bearing organic materials for an extended period of time, organic materials must be eliminated. In addition, carbide and nitride fuels must be converted into oxides for safe storage in order to prevent the exothermal reaction of these fuels with oxygen/moisture in air. A survey of the literature on the stabilization treatment of Pu-bearing organic materials confirmed that organic materials can be decomposed and removed by heating at 950  C (1223.15 K) or greater in air. Furthermore, based on the calculated thermodynamic parameters of oxidation reaction of carbide and nitride fuels in air, it was estimated that these fuels would be oxidized in air at 950
C (1223.15 K) or greater in air. Furthermore, based on the calculated thermodynamic parameters of oxidation reaction of carbide and nitride fuels in air, it was estimated that these fuels would be oxidized in air at 950  C because the equilibrium oxygen partial pressure in the oxidation reaction at 950
C because the equilibrium oxygen partial pressure in the oxidation reaction at 950  C was lower than 2.1
C was lower than 2.1 10
10 Pa (oxygen partial pressure in air). Therefore, it was decided to stabilize Pu-bearing organic materials by heating at 950
Pa (oxygen partial pressure in air). Therefore, it was decided to stabilize Pu-bearing organic materials by heating at 950  C in air to remove the organic materials and oxidize the carbide and nitride fuels. As a mock-up test to remove the organic materials, thin sheets of epoxy resin were heated in air. The changes in appearance and weight before and after heating in air showed that organic materials can be removed. After the mock-up test, Pu-bearing organic materials were also stabilized by heating in the similar condition.
C in air to remove the organic materials and oxidize the carbide and nitride fuels. As a mock-up test to remove the organic materials, thin sheets of epoxy resin were heated in air. The changes in appearance and weight before and after heating in air showed that organic materials can be removed. After the mock-up test, Pu-bearing organic materials were also stabilized by heating in the similar condition.

Watanabe, Masashi; Seki, Takayuki*
Materials Science & Engineering B, 272, p.115369_1 - 115369_6, 2021/10
Times Cited Count:1 Percentile:7.92(Materials Science, Multidisciplinary)The effect of oxygen non-stoichiometry on the initial sintering behavior of CeO was investigated. It was found that the initial sintering of the stoichiometric and hypo-stoichiometric composition was controlled by the grain boundary diffusion. The activation energies of cation diffusion were derived from initial sintering data. Moreover, it is suggested that the cation diffusion was caused by a vacancy mechanism.
was investigated. It was found that the initial sintering of the stoichiometric and hypo-stoichiometric composition was controlled by the grain boundary diffusion. The activation energies of cation diffusion were derived from initial sintering data. Moreover, it is suggested that the cation diffusion was caused by a vacancy mechanism.
Kihara, Yoshiyuki; Tanaka, Kosuke; Koyama, Shinichi; Yoshimochi, Hiroshi; Seki, Takayuki; Katsuyama, Kozo
NEA/NSC/R(2017)3, p.341 - 350, 2017/11
In order to investigate the effect of the addition of americium to MOX fuels on the irradiation behaviour, the "Am-1" program is being conducted at the JAEA. The Am-1 program consists of two short-term irradiation tests of 10-min and 24-h irradiation periods, and a steady-state irradiation test. The short-term irradiation tests and their post irradiation examinations (PIEs) have been successfully completed. To date, the data for PIE of the Am-MOX fuels focused on the microstructural evolution and redistribution behaviour of Am at the initial stage of irradiation have been obtained and reported. In this paper, the results obtained from the Am-1 program are reviewed and detailed descriptions of the fabrication and inspection techniques for the Am-MOX fuels prepared for the program are provided. PIE data for the Am-MOX fuels at the initial stage of irradiation have been accumulated. In this paper, unpublished PIE data for the Am-MOX fuels are also presented.
Tanaka, Kosuke; Miwa, Shuhei; Sato, Isamu; Hirosawa, Takashi; Sekine, Shinichi; Seki, Takayuki*; Tokoro, Daishiro*; Obayashi, Hiroshi; Koyama, Shinichi
JAEA-Research 2013-022, 62 Pages, 2014/01
In order to establish the method for heating tests focused on the fission product release resulting from the high temperature chemical interaction between fuel and cladding material and to obtain the novel data on fission product release behaviors, the heating test was carried out with irradiate MOX fuel pellet and cladding.
Sato, Isamu; Seki, Takayuki*; Ishi, Yohei; Mondo, Kenji; Yoshimochi, Hiroshi; Tanaka, Kenya
JAEA-Research 2007-013, 63 Pages, 2007/03
In air atmosphere, the weight of Am-MOX fuel relatively rapidly increased, which change rate strongly depended on the initial O/M ratio. The lower initial O/M ratio is, the higher the rate is. However, for the other MOX fuel containing little Am, the equivalent behavior have been observed, which indicated that not only Am but also the property of law material powder affected the behavior. The X-ray diffraction pattern change as time goes by was observed, which was a evidence that the O/M ratio change might arise from crystallographic one. The rate of O/M ratio change is a function of water vapor pressure in the atmosphere. If the water vapor pressure would is set to be quite low (ex.  1ppm), the O/M ratio change could effectively been avoided. On a model basis of Am(III) and U(V) existence, we could explain the O/M ratio dependence of the lattice parameter of Am-MOX fuel near O/M ratio, 2.00 better.
1ppm), the O/M ratio change could effectively been avoided. On a model basis of Am(III) and U(V) existence, we could explain the O/M ratio dependence of the lattice parameter of Am-MOX fuel near O/M ratio, 2.00 better.
 by an in-cell remote process
by an in-cell remote processYoshimochi, Hiroshi; Ishi, Yohei; Seki, Takayuki*; Mondo, Kenji; Sekine, Shinichi*; Koyama, Shinichi
Saikuru Kiko Giho, (28), p.9 - 20, 2005/09
An in-cell remote fabrication technique has been developed for MOX fuel pellets containing 3 and 5% americium (Am-MOX) at the Alpha-gamma Facility (AGF) in O-arai Engineering Center. A series of fuel pellet and the pin fabrication apparatuses were systematically installed in hot cell to make fabrication flow easier. After cold run and some modifications, they were remotely controlled from a panel in the operation room outside the hot cell as much as possible. From a preliminary UO2 pellet test and consequently plutonium pellet fabrication run, actual range of ball milling time, pressing and sintering condition were focused for Am-MOX pellet fabrication. As the next step, moisturized atmosphere was found out to remove the heterogeneity structure of 5% Am-MOX pellet. Finally, we established an optimized fabrication condition of 5% Am-MOX pellet sintered at 1700 C for 3h in an atmosphere of 5% H2-95% Ar with total moisture of 2000 ppm. Moreover it is important that the atmosphere has to be changed to dry gas at 800
C for 3h in an atmosphere of 5% H2-95% Ar with total moisture of 2000 ppm. Moreover it is important that the atmosphere has to be changed to dry gas at 800 C during cool down.
C during cool down.
Miwa, Shuhei; Osaka, Masahiko; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*; Sekine, Shinichi*
JNC TN9400 2005-023, 43 Pages, 2005/04
The effect of oxygen potential on the sintering behavior of MOX fuel containing Am (Am-MOX) was investigated. Green pellets of Am-MOX were prepared by a conventional powder metallurgical technique. For Am-MOX fuel pellets sintered at various oxygen potential conditions, density measurement, microstructural observation and elements analyses by EPMA were performed High density pellets having good structure were obtained due to oxygen potential change of sintering atmosphere from high oxygen potential to low oxygen potential at 800 C in the cooling process.For the pellets sintered at -520 kJ/mol, -390 kJ/mol and -340 kJ/mol, the sintered density increases with increase of oxygen potential up to -390 kJ/mol (threshold oxygen potential), then decreases above the threshold oxygen potential. This tendency is similar to that observed in the (U,Gd)O
C in the cooling process.For the pellets sintered at -520 kJ/mol, -390 kJ/mol and -340 kJ/mol, the sintered density increases with increase of oxygen potential up to -390 kJ/mol (threshold oxygen potential), then decreases above the threshold oxygen potential. This tendency is similar to that observed in the (U,Gd)O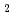 system. The differences of sintering behavior for Am-MOX pellets which were observed by changing the oxygen potential were attributable to the difference of pore structure, which was supposed to be caused by the valence state of Am in the oxides. On the other hands, the grain size of Am-MOX pellet sintered at -520 kJ/mol was almost the same as that at -390 kJ/mol. Homogeneous distribution of U, Pu and Am was obtained at pellets sintered both -520 and -390 kJ/mol in these sintering conditions. For the pellets sintered at 1500
system. The differences of sintering behavior for Am-MOX pellets which were observed by changing the oxygen potential were attributable to the difference of pore structure, which was supposed to be caused by the valence state of Am in the oxides. On the other hands, the grain size of Am-MOX pellet sintered at -520 kJ/mol was almost the same as that at -390 kJ/mol. Homogeneous distribution of U, Pu and Am was obtained at pellets sintered both -520 and -390 kJ/mol in these sintering conditions. For the pellets sintered at 1500 C , 1600
C , 1600 C , 1700
C , 1700 C , the high dense pellets are obtained, therefore This results shows the the possibility of fabrication of good fuel pellets at lower temperature than 1700
C , the high dense pellets are obtained, therefore This results shows the the possibility of fabrication of good fuel pellets at lower temperature than 1700 C
C

Osaka, Masahiko; Miwa, Shuhei; Mondo, Kenji; Ozaki, Yoko; Ishi, Yohei; Yoshimochi, Hiroshi; Seki, Takayuki*; Sekine, Shinichi*; Ishida, Takashi*; Tanaka, Kenya
JNC TN9400 2005-002, 40 Pages, 2005/03
An experimental investigation for the phase relation of (U,Pu,Am)O was performed with XRD, ceramography and DTA. Although lattice parameter tended to increase with increases of Am content and O/M ratio, its slope differed from that of (U,Pu)O
was performed with XRD, ceramography and DTA. Although lattice parameter tended to increase with increases of Am content and O/M ratio, its slope differed from that of (U,Pu)O . A drastic structural change was observed around O/M=1.98. Besides, many DTA peaks, which could never be seen in the case of (U,Pu)O
. A drastic structural change was observed around O/M=1.98. Besides, many DTA peaks, which could never be seen in the case of (U,Pu)O , were observed above O/M=1.98.These results were interpreted with a hypothesis that all Am were trivalent and equivalent amount of U became pentavalent. The dependence of lattice parameter on Am content could be expressed well by using a model with ionic radii of each element. Also the structural change around O/M=1.98 could be explained as caused by valence states of each element. It was concluded from these interpretation that all Am in (U,Pu,Am)O
, were observed above O/M=1.98.These results were interpreted with a hypothesis that all Am were trivalent and equivalent amount of U became pentavalent. The dependence of lattice parameter on Am content could be expressed well by using a model with ionic radii of each element. Also the structural change around O/M=1.98 could be explained as caused by valence states of each element. It was concluded from these interpretation that all Am in (U,Pu,Am)O were likely to exist as trivalent state.
were likely to exist as trivalent state.
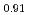 Am
Am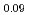 )O
)O
Osaka, Masahiko; Mondo, Kenji; Ishi, Yohei; Tanaka, Kenya; Seki, Takayuki*; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*
no journal, ,
Oxygen potential of (Pu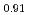 Am
Am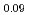 )O
)O were measured by thermogravimetry using H
were measured by thermogravimetry using H O/H
O/H gas equiribrium, as a fundamental part of investigation on phase relation for low-decontaminated fuel.
gas equiribrium, as a fundamental part of investigation on phase relation for low-decontaminated fuel.
Sato, Isamu; Ishi, Yohei; Mondo, Kenji; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*
no journal, ,
Wieght changes of MOX fuels containing Am were measured during stored in a cell box. We observed that the O/M ratios of these fuels containing Am changed relatively rapidly.
Yoshimochi, Hiroshi; Ishi, Yohei; Tanaka, Kenya; Sekine, Shinichi*; Seki, Takayuki*
no journal, ,
no abstracts in English
Mondo, Kenji; Ishi, Yohei; Sato, Isamu; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*
no journal, ,
no abstracts in English
Sato, Isamu; Ishi, Yohei; Mondo, Kenji; Tanaka, Kenya; Seki, Takayuki*; Ishida, Takashi*
no journal, ,
A dependence of O/M ratio change behavior on atmosphere was evaluated, then these results indicated that the change rate of the O/M ratio depended on the concentration of water.
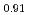 Am
Am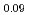 )O
)O
Miwa, Shuhei; Osaka, Masahiko; Mondo, Kenji; Ishi, Yohei; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*; Sekine, Shinichi*; Kurosaki, Ken*; Uno, Masayoshi*; et al.
no journal, ,
An experimental investigation on phase relation of (Pu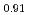 Am
Am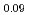 )O
)O was carried out by X-ray diffraction (XRD) analysis, ceramography and Differential Thermal Analysis (DTA) for (Pu
was carried out by X-ray diffraction (XRD) analysis, ceramography and Differential Thermal Analysis (DTA) for (Pu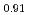 Am
Am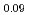 )O
)O having different O/M ratios from 1.90 to 2.00.
having different O/M ratios from 1.90 to 2.00.
Miwa, Shuhei; Osaka, Masahiko; Tanaka, Kosuke; Ishi, Yohei; Tanaka, Kenya; Sekine, Shinichi*; Seki, Takayuki*
no journal, ,
R&D of advanced fuel containing minor actinide for use in fast reactors is described related to the composite fuel with MgO matrix. Fabrication tests of MgO composite fuels containing Am were done by a practical process that could be adapted to the presently used commercial manufacturing technology. Am-containing MgO composite fuels having good characteristics, i.e., having no defects, a high density, a homogeneous dispersion of host phase, were obtained.

Miwa, Shuhei; Osaka, Masahiko; Tanaka, Kosuke; Seki, Takayuki*
no journal, ,
For the evaluation of phase relation and irradiation behavior of low-decontaminated fuel and heterogeneous fuel, the oxygen potential of (Pu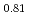 Am
Am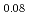 Nd
Nd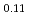 )O
)O was investigated by the thermogravity analysis in various atmospheres which was adjusted by changing the ratio of H
was investigated by the thermogravity analysis in various atmospheres which was adjusted by changing the ratio of H O/H
O/H , CO
, CO /H
/H and dilution ratio of O
and dilution ratio of O .
.
Miwa, Shuhei; Osaka, Masahiko; Sato, Isamu; Hirosawa, Takashi; Tanaka, Kosuke; Sekine, Shinichi*; Ishida, Takashi*; Seki, Takayuki*; Kashimura, Naoki*
no journal, ,
no abstracts in English
Tanaka, Kosuke; Sato, Isamu; Hirosawa, Takashi; Seki, Takayuki*; Kashimura, Naoki*; Kurosaki, Ken*; Tokushima, Kazuyuki*; Muta, Hiroaki*; Oishi, Yuji*; Yamanaka, Shinsuke*
no journal, ,
Complex uranium or plutonium oxides with alkaline-earth metals were prepared by using conventional powder metallurgy and its thermophysical properties were evaluated.
Miwa, Shuhei; Usuki, Toshiyuki; Sato, Isamu; Hirosawa, Takashi; Tanaka, Kosuke; Osaka, Masahiko; Seki, Takayuki*; Kashimura, Naoki*
no journal, ,
no abstracts in English
Miwa, Shuhei; Usuki, Toshiyuki; Sato, Isamu; Hirosawa, Takashi; Tanaka, Kosuke; Osaka, Masahiko; Seki, Takayuki*
no journal, ,
Thermal conductivies of MgO-based inert matrix fuels containing PuO using the asbestos waste-derived ceramics as a sintering additive were experimentally investigated. Thermal diffusivities of fuels were measured by laser flush method. The effects of the MgO-SiO
using the asbestos waste-derived ceramics as a sintering additive were experimentally investigated. Thermal diffusivities of fuels were measured by laser flush method. The effects of the MgO-SiO compound additives addition on the thermal conductivity were discussed.
compound additives addition on the thermal conductivity were discussed.