Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tobitsuka, Sachiko; Iijima, Kazuki; Kohara, Yukitoshi*
Proceedings of 29th International Symposium of Scientific Basis for Nuclear Waste Management (MRS 2005), 0 Pages, 2005/09
The influence of humic acid for the solubility of tetravalent neptunium was investigated under reducing condition, by comparing at various pH. All of experiments were carried in an argon glove-box except analyses. At pH5, 8 and 9, at 20-24 degree C, Np solution in 1E-5 or 1E-3 mol/l was mixed with 0, 5-1,000 mg/l of purified Aldrich humic acid (PAHA). The ionic strength was 0.25 which was brought by 0.1 mol/l NaClO and 0.05 mol/l Na
and 0.05 mol/l Na S
S O
O added as a reductant to keep Np in tetravalent. The concentration of Np was quantified periodically by alpha spectrometry or ICP-MS. With the acid-base titration, analyzed by NICA-Donnan model which assumes the carboxyl group and the phenolic ydroxyl group as two main functional groups, the charge density of PAHA was 2.84, 4.36, 4.78 meq/g at pH5, 8, 9, respectively. In all cases, the total Np concentration ([Np]t) increases with increasing PAHA concentration. In the case of 1E-5 mol/l of initial Np concentration ([Np]
added as a reductant to keep Np in tetravalent. The concentration of Np was quantified periodically by alpha spectrometry or ICP-MS. With the acid-base titration, analyzed by NICA-Donnan model which assumes the carboxyl group and the phenolic ydroxyl group as two main functional groups, the charge density of PAHA was 2.84, 4.36, 4.78 meq/g at pH5, 8, 9, respectively. In all cases, the total Np concentration ([Np]t) increases with increasing PAHA concentration. In the case of 1E-5 mol/l of initial Np concentration ([Np]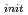 especially, [Np]t increases almost up to [Np]
especially, [Np]t increases almost up to [Np]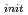 in 50 mg/l PAHA at pH8, 9, or 25 mg/l at pH5. Even in the case of PAHA 5 mg/l, [Np]t becomes one order or more higher than that in the absence of PAHA. The apparent stability constant
in 50 mg/l PAHA at pH8, 9, or 25 mg/l at pH5. Even in the case of PAHA 5 mg/l, [Np]t becomes one order or more higher than that in the absence of PAHA. The apparent stability constant
Tobitsuka, Sachiko; Kikuchi, Hirohito*; Matsumoto, Kazuhiro*; Iijima, Kazuki; Sato, Haruo
JNC TN8400 2005-019, 65 Pages, 2005/08
The permeation behavior of organic matter through a compacted bentonite was evaluated in low and high ionic strength condition. The concentration of organic matters eluting from bentonite was analyzed. Polyacrylic acid solutions (average molecular weight:15,000 or 450,000) were permeated during maximum 140 days to the compacted bentonite (Kunigel V1, 100%) at dry density 1.2 g/cm . In low ionc strength condition, polyacrylic acid sodium (MWave: 15,000) was detected after 70 days in the permeated solution. In high ionic strength condition, it was found after 52 days. Polyacrylic acid (MWave: 450,000) didn't permeate in the both of system. It indicates that organic matters less than MWave: 450,000 aren't filtrated by bentonite. It is supposed that the organic compound which can change its conformation to a chain-like structure, are not likely to be filtrated, even if its molecular weight is more than 15,000. In low ionic strength condition, the concentration of eluting organic carbon from bentonite is 7.4-12.5 mg /l. In high ionic strength condition it is 4.2-6.5 mg /l.
. In low ionc strength condition, polyacrylic acid sodium (MWave: 15,000) was detected after 70 days in the permeated solution. In high ionic strength condition, it was found after 52 days. Polyacrylic acid (MWave: 450,000) didn't permeate in the both of system. It indicates that organic matters less than MWave: 450,000 aren't filtrated by bentonite. It is supposed that the organic compound which can change its conformation to a chain-like structure, are not likely to be filtrated, even if its molecular weight is more than 15,000. In low ionic strength condition, the concentration of eluting organic carbon from bentonite is 7.4-12.5 mg /l. In high ionic strength condition it is 4.2-6.5 mg /l.
Iijima, Kazuki; Tobitsuka, Sachiko; Kohara, Yukitoshi*
JNC TN8400 2004-005, 46 Pages, 2004/04
Complexation behavior between humic acid (HA), which is one of the natural organic matter in gourndwater, and tetravalent neptunium (Np(IV)) was investigated by batch type copmlexation experiments and solvent extraction by TTA xylene, focused on the effect concentration and molecular weight of HA. In addition, proton dissociation behavior from HA was evaluated by acid-base titration and its results was used for the calculation of stability constant of Np(IV) and HA.
Tobitsuka, Sachiko; Kohara, Yukitoshi*; Iijima, Kazuki; Sato, Haruo
JNC TN8400 2003-018, 53 Pages, 2003/06
Humic substances which are humic acid, fulvic acid and humin, exist in the groundwater and the pore water inside of compacted bentonite as the natural organic matters. These are considered that these make increase the solubility of radionuclieds, or decre