Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tobita, Minoru*; Konda, Miki; Omori, Takeshi*; Nabatame, Tsutomu*; Onizawa, Takashi*; Kurosawa, Katsuaki*; Haraga, Tomoko; Aono, Ryuji; Mitsukai, Akina; Tsuchida, Daiki; et al.
JAEA-Data/Code 2022-007, 40 Pages, 2022/11
Radioactive wastes generated from nuclear research facilities in Japan Atomic Energy Agency are planning to be buried in the near surface disposal field. Therefore, it is required to establish the method to evaluate the radioactivity concentrations of radioactive wastes until the beginning of disposal. In order to contribute to this work, we collected and analyzed concrete, ash, ceramic and brick samples generated from JRR-3, JRR4 and JRTF facilities. In this report, we summarized the radioactivity concentrations of 24 radionuclides ( H,
H,  C,
C, 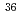 Cl,
Cl, 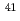 Ca,
Ca,  Co,
Co, 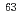 Ni,
Ni,  Sr,
Sr, 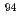 Nb,
Nb,  Tc,
Tc, 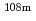 Ag,
Ag,  I,
I,  Cs,
Cs,  Ba,
Ba, 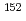 Eu,
Eu, 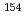 Eu,
Eu, 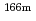 Ho,
Ho,  U,
U,  U,
U,  Pu,
Pu,  Pu,
Pu,  Pu,
Pu,  Am,
Am,  Am,
Am,  Cm) which were obtained from radiochemical analysis of the samples in fiscal years 2020-2021.
Cm) which were obtained from radiochemical analysis of the samples in fiscal years 2020-2021.
Nishimura, Shoichiro*; Torii, Hiroyuki*; Fukao, Yoshinori*; Ito, Takashi; Iwasaki, Masahiko*; Kanda, Sotaro*; Kawagoe, Kiyotomo*; Kawall, D.*; Kawamura, Naritoshi*; Kurosawa, Noriyuki*; et al.
Physical Review A, 104(2), p.L020801_1 - L020801_6, 2021/08
Times Cited Count:13 Percentile:84.11(Optics)Oguri, Hidetomo; Hasegawa, Kazuo; Ito, Takashi; Chishiro, Etsuji; Hirano, Koichiro; Morishita, Takatoshi; Shinozaki, Shinichi; Ao, Hiroyuki; Okoshi, Kiyonori; Kondo, Yasuhiro; et al.
Proceedings of 11th Annual Meeting of Particle Accelerator Society of Japan (Internet), p.389 - 393, 2014/10
no abstracts in English
Sakanaka, Shogo*; Akemoto, Mitsuo*; Aoto, Tomohiro*; Arakawa, Dai*; Asaoka, Seiji*; Enomoto, Atsushi*; Fukuda, Shigeki*; Furukawa, Kazuro*; Furuya, Takaaki*; Haga, Kaiichi*; et al.
Proceedings of 1st International Particle Accelerator Conference (IPAC '10) (Internet), p.2338 - 2340, 2010/05
Future synchrotron light source using a 5-GeV energy recovery linac (ERL) is under proposal by our Japanese collaboration team, and we are conducting R&D efforts for that. We are developing high-brightness DC photocathode guns, two types of cryomodules for both injector and main superconducting (SC) linacs, and 1.3 GHz high CW-power RF sources. We are also constructing the Compact ERL (cERL) for demonstrating the recirculation of low-emittance, high-current beams using above-mentioned critical technologies.
Ono, Masao; Sueyoshi, Masanori*; Okayasu, Satoru; Hao, T.; Esaka, Fumitaka; Osawa, Takahito; Iguchi, Yusuke*; Mashimo, Tsutomu
Review of Scientific Instruments, 80(8), p.083908_1 - 083908_6, 2009/08
Times Cited Count:3 Percentile:17.76(Instruments & Instrumentation)A prototype rotor with 2 grooves for the multi-stage centrifugal isotope separation in solid state was developed to test a new idea. This idea is based on the sedimentation of constitutional atoms in solid. In the performance test using indium specimen, it is verified that the developed rotor can receive all injected molten-indium droplets from an automatic raw-material feeding system even at the high rotational speed of 97,000 r.p.m. without the loss of rotational stability, and the received indium specimens can be transferred in/between 2 grooves through the plastic flow under the influence of strong centrifugal force even in the solid state. The isotope ratio of centrifuged indium specimens was analyzed employing the Secondary Ion Mass Spectrometry (SIMS), and it is confirmed that intended isotope separation by the centrifugation is realized in the solid state.
Ebisawa, Hiroyuki; Hanakawa, Hiroki; Asano, Norikazu; Kusunoki, Hidehiko; Yanai, Tomohiro; Sato, Shinichi; Miyauchi, Masaru; Oto, Tsutomu; Kimura, Tadashi; Kawamata, Takanori; et al.
JAEA-Technology 2009-030, 165 Pages, 2009/07
The condition of facilities and machinery used continuously were investigated before the renewal work of JMTR on FY 2007. The subjects of investigation were reactor building, primary cooling system tanks, secondary cooling system piping and tower, emergency generator and so on. As the result, it was confirmed that some facilities and machinery were necessary to repair and others were used continuously for long term by maintaining on the long-term maintenance plan. JMTR is planed to renew by the result of this investigation.
Ono, Masao; Iguchi, Yusuke*; Okayasu, Satoru; Esaka, Fumitaka; Kobayashi, Katsura*; Hao, T.; Bagum, R.*; Osawa, Takahito; Fujii, Kimio; Nakamura, Eizo*; et al.
Defect and Diffusion Forum, 289-292, p.63 - 68, 2009/04
The atomic-scale graded structure of In-Pb alloy was formed by an ultracentrifuge experiment under conditions that a gravitational field of 0.81 10
10 G for 100 hours at 150
G for 100 hours at 150  C in solid state in our previous study. The isotope ratio measurements were performed on the centrifuged sample with secondary ion mass spectrometer (SIMS).
C in solid state in our previous study. The isotope ratio measurements were performed on the centrifuged sample with secondary ion mass spectrometer (SIMS).  Pb/
Pb/ Pb and
Pb and 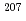 Pb/
Pb/ Pb isotope ratio changed with negative gradient in the direction of centrifugal force approximately 1.5% and 0.8%, respectively. And three-isotope diagram of
Pb isotope ratio changed with negative gradient in the direction of centrifugal force approximately 1.5% and 0.8%, respectively. And three-isotope diagram of  Pb/
Pb/ Pb versus
Pb versus 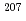 Pb/
Pb/ Pb proved that the isotope fluctuation depends on isotopic mass difference. These results showed that the strong gravitational field not only affected on the inter-diffusion but also on self-diffusion in this alloy as causing isotope fractionation effect, and the isotope fractionation was dependent on mass-difference.
Pb proved that the isotope fluctuation depends on isotopic mass difference. These results showed that the strong gravitational field not only affected on the inter-diffusion but also on self-diffusion in this alloy as causing isotope fractionation effect, and the isotope fractionation was dependent on mass-difference.
Osawa, Takahito; Ono, Masao; Esaka, Fumitaka; Okayasu, Satoru; Iguchi, Yusuke*; Hao, T.; Magara, Masaaki; Mashimo, Tsutomu
EPL; A Letters Journal Exploring the Frontiers of Physics, 85(6), p.64001_1 - 64001_5, 2009/03
Times Cited Count:6 Percentile:41.57(Physics, Multidisciplinary)Pure tin metals were centrifuged at 1
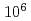
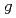 and at 220
and at 220  C for 100 hours, 0.40
C for 100 hours, 0.40
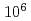
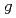 at 220-230
at 220-230  C for 24 hours, and 0.25
C for 24 hours, and 0.25
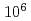
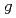 at 220
at 220  C for 24 hours. Their isotopic compositions were measured by a secondary ion mass spectrometer (SIMS).
C for 24 hours. Their isotopic compositions were measured by a secondary ion mass spectrometer (SIMS). 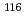 Sn/
Sn/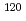 Sn and
Sn and 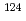 Sn/
Sn/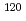 Sn ratios of the 1.02
Sn ratios of the 1.02
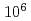
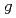 sample were considerably different than the initial compositions, and the magnitude of isotopic fractionation reached 2.6%. A three-isotope diagram of
sample were considerably different than the initial compositions, and the magnitude of isotopic fractionation reached 2.6%. A three-isotope diagram of 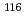 Sn/
Sn/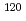 Sn versus
Sn versus 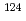 Sn/
Sn/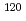 Sn shows conclusively that isotopic fractionation caused by a gravitational field depended only on isotopic mass.
Sn shows conclusively that isotopic fractionation caused by a gravitational field depended only on isotopic mass.
Ono, Masao; Iguchi, Yusuke*; Okayasu, Satoru; Esaka, Fumitaka; Kobayashi, Katsura*; Hao, T.; Bagum, R.*; Osawa, Takahito; Fujii, Kimio; Nakamura, Eizo*; et al.
Journal of Nuclear Science and Technology, 45(Suppl.6), p.108 - 110, 2008/09
Times Cited Count:1 Percentile:10.01(Nuclear Science & Technology)The atomic-scale graded structure of In-Pb alloy was formed by an ultracentrifuge experiment under conditions that a gravitational field of 810,000 G for 100 hours at 150 C (solid state) in our previous study. The isotope fluctuation on this sample was measured using secondary ion mass spectrometer (SIMS). The ratio both
C (solid state) in our previous study. The isotope fluctuation on this sample was measured using secondary ion mass spectrometer (SIMS). The ratio both  Pb/
Pb/ Pb and
Pb and 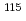 In/
In/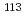 In changed with positive gradient in the direction of centrifugal force approximately 1.2%. These isotope fluctuations were larger than that of starting state of natural state (In
In changed with positive gradient in the direction of centrifugal force approximately 1.2%. These isotope fluctuations were larger than that of starting state of natural state (In 0.2%, Pb
0.2%, Pb 0.1%). These show that the sedimentation of isotopes occurred by solid centrifugation in this alloy, although achieved concentration gradients were small.
0.1%). These show that the sedimentation of isotopes occurred by solid centrifugation in this alloy, although achieved concentration gradients were small.
Fujita, Hideki*; Negishi, Kumi*; Osawa, Tsutomu*; Honda, Akira
Semento, Konkurito Rombunshu, (61), p.262 - 269, 2008/02
The influence of sodium nitrate on dissolution of cement hydrates was investigated by flow through experiment. Dissolution of portlandite was accelerated in NaNO solution of 1 mol dm
solution of 1 mol dm than in ion exchanged water. The acceleration of portlandite dissolution attributed to impact of ionic strength on the activities of aqueous species, because Ca concentration in 1 mol kg
than in ion exchanged water. The acceleration of portlandite dissolution attributed to impact of ionic strength on the activities of aqueous species, because Ca concentration in 1 mol kg NaNO
NaNO solution equilibrated with portlandite was estimated to be 1.5 times as high as that in pure water. After flow through experiment, compressive strength fell down and porosity was higher than blank test using pure water. However low permeabilities were contradictory kept during NaNO
solution equilibrated with portlandite was estimated to be 1.5 times as high as that in pure water. After flow through experiment, compressive strength fell down and porosity was higher than blank test using pure water. However low permeabilities were contradictory kept during NaNO solution flowing. Dense existence of Na was possible to cause the low permeability during NaNO
solution flowing. Dense existence of Na was possible to cause the low permeability during NaNO solution flowing.
solution flowing.
Tachibana, Haruo; Kikuchi, Masamitsu; Sekita, Tsutomu; Yamaguchi, Takenori; Oeda, Mikihiro*; Kurosawa, Naohiro*
JAERI-Data/Code 2004-010, 19 Pages, 2004/06
This report is a revised edition of "Isopleths of Surface Air Concentration and Surface Air Absorbed Dose Rate due to a Radioactive Cloud Released from a Stack(II)"(JAERI-M 90-206) and based on the revised Nuclear Safety Guidelines reflected the ICRP1990 Recommendation. Characteristics of this report are the use of Air Karma Rate (Gy/h) unit instead of Air Absorbed Dose Rate (Gy/h) unit, and the records of isopleths of surface air concentration and surface air karma rate on CD-ROM. These recorded data on CD-ROM can be printed out on paper and/or pasted on digital map by personal computer.
Osawa, Hideaki; Sato, Tsutomu*; Sakai, Ryutaro; Osawa, Hideaki; Kodama, Toshio*
Nihon Suimon Kagakkai-Shi, 29(1), p.13 - 24, 1999/00
None
Ono, Masao; Iguchi, Yusuke*; Okayasu, Satoru; Esaka, Fumitaka; Kobayashi, Katsura*; Hao, T.; Bagum, R.*; Osawa, Takahito; Fujii, Kimio; Nakamura, Eizo*; et al.
no journal, ,
The atomic-scale graded structure of In-Pb alloy was formed by an ultracentrifuge experiment under conditions that a gravitational field of 0.81 10
10 G for 100 hours at 150
G for 100 hours at 150  C in solid state in our previous study. The isotope ratio measurements were performed on the centrifuged sample with secondary ion mass spectrometer (SIMS, CAMECA IMS-6f). Both
C in solid state in our previous study. The isotope ratio measurements were performed on the centrifuged sample with secondary ion mass spectrometer (SIMS, CAMECA IMS-6f). Both 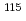 In/
In/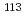 In and
In and  Pb/
Pb/ isotope ratio changed with negative gradient in the direction of centrifugal force approximately 1.2% showing the tendency that the heavy
isotope ratio changed with negative gradient in the direction of centrifugal force approximately 1.2% showing the tendency that the heavy 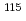 In isotope (
In isotope ( Pb isotope) abundance increased and light
Pb isotope) abundance increased and light 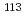 In isotope (
In isotope ( Pb isotope) abundance decreased in the direction of centrifugal force. This showed that the isotope fractionation effect due to sedimentation of atoms occurred in this alloy by ultracentrifuge experiment.
Pb isotope) abundance decreased in the direction of centrifugal force. This showed that the isotope fractionation effect due to sedimentation of atoms occurred in this alloy by ultracentrifuge experiment.
Osawa, Takahito; Ono, Masao; Esaka, Fumitaka; Okayasu, Satoru; Iguchi, Yusuke*; Hao, T.; Mashimo, Tsutomu
no journal, ,
Pure tin metals were centrifuged at 1,0200,000G and at 220 C for 100 hours, 400,000 G at 220-230
C for 100 hours, 400,000 G at 220-230 C for 24 hours, and 250,000G at 220
C for 24 hours, and 250,000G at 220 C for 24 hours. Their isotopic compositions were measured by a secondary ion mass spectrometer (SIMS). 116Sn/120Sn and 124Sn/120Sn ratios of the 1,0200,000G sample were considerably different than the initial compositions, and the magnitude of isotopic fractionation reached 2.6%. A three-isotope diagram of 116Sn/120Sn versus 124Sn/120Sn shows conclusively that isotopic fractionation caused by a gravitational field depended on isotopic mass.
C for 24 hours. Their isotopic compositions were measured by a secondary ion mass spectrometer (SIMS). 116Sn/120Sn and 124Sn/120Sn ratios of the 1,0200,000G sample were considerably different than the initial compositions, and the magnitude of isotopic fractionation reached 2.6%. A three-isotope diagram of 116Sn/120Sn versus 124Sn/120Sn shows conclusively that isotopic fractionation caused by a gravitational field depended on isotopic mass.
Ono, Masao; Hao, T.; Okayasu, Satoru; Esaka, Fumitaka; Osawa, Takahito; Iguchi, Yusuke*; Mashimo, Tsutomu
no journal, ,
no abstracts in English
Ichikawa, Mariko*; Iizuka, Tomoko*; Gamo, Emi*; Kobori, Emiko*; Shibuya, Michiko*; Shibosawa, Hisako*; Chiba, Etsuko*; Yokoyama, Tsutomu*; Fukutomi, Fumitake*; Todoriki, Setsuko*; et al.
no journal, ,
no abstracts in English
Osawa, Takahito; Ono, Masao; Esaka, Fumitaka; Okayasu, Satoru; Mashimo, Tsutomu*
no journal, ,
no abstracts in English
Osawa, Takahito; Ono, Masao; Esaka, Fumitaka; Okayasu, Satoru; Mashimo, Tsutomu*
no journal, ,
no abstracts in English
Ono, Masao; Okayasu, Satoru; Iguchi, Yusuke*; Esaka, Fumitaka; Ishitsuka, Etsuo; Osawa, Takahito; Ogata, Yudai; Mashimo, Tsutomu*
no journal, ,
Kobayashi, Yutaro*; Osawa, Norihisa*; Haga, Kazuko*; Kaneda, Yoshihisa*; Chaerun Raudhatul, I.*; Sato, Tsutomu*; Taniguchi, Takumi; Kuroki, Ryoichiro; Osugi, Takeshi
no journal, ,
no abstracts in English