Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Shoji, Mizuki*; Kurihara, Kensuke*; Lobzenko, I.; Tsuru, Tomohito; Serizawa, Ai*
Keikinzoku, 74(12), p.535 - 545, 2024/12
While Plate-like Guinier-Preston (GP) zones are formed during aging process in Al-Cu alloys, spherical nanocluster formation occurs in the early stage of aging in Al-Mg-Si alloys. Unlike well-known GP (I) zone in Al-Cu, there is no specific configurations within the nanocluster. However, the solute concentration and local configuration should play decisive role in subsequent formation of precipitations. In the present study, the first-principles calculations were performed to investigate the factors determining the stable shape during the formation process of GP zones and clusters in Al-Cu and Al-Mg-Si alloys. As a result of formation energy of three-body bonds, the Cu-Cu-Cu triplet with the bond angle of 90deg was the most stable. Monte Carlo simulations with newly developed machine-learning potential were then performed, and consequently the segregation of Cu atom formed with bond angle of 90deg are observed more frequently. In contrast, three-body triplet in Al-Mg-Si alloy was most stable without any specific directional anisotropy, when the bond angle was 60deg, resulting in the formation of spherical nanoclusters. These results suggest that the intrinsic feature of the stability of local bonding dominates the shape of GP zones and nanoclusters, in which planar- or spherical-like cluster is formed.
Arai, Yoichi; Watanabe, So; Watanabe, Masayuki; Arai, Tsuyoshi*; Katsuki, Kenta*; Agou, Tomohiro*; Fujikawa, Hisaharu*; Takeda, Keisuke*; Fukumoto, Hiroki*; Hoshina, Hiroyuki*; et al.
Nuclear Instruments and Methods in Physics Research B, 554, p.165448_1 - 165448_10, 2024/09
Times Cited Count:0 Percentile:0.00(Instruments & Instrumentation)Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 40(2), p.347 - 352, 2024/02
Times Cited Count:2 Percentile:23.37(Chemistry, Analytical)The Eu(III) distribution mechanism in single extractant-impregnated polymer-layered silica particle in a complex solution containing multiple lanthanide ions was investigated using fluorescence microspectroscopy. The rate-determining step was the reaction of Eu(III) with the two extractant molecules. The obtained mechanism and rate constants agreed with those of the single-ion distribution system, in which Eu(III) was distributed to the particles in the Eu(III) solution.
 CoSi
CoSiYamauchi, Hiroki; Sari, D. P.*; Yasui, Yukio*; Sakakura, Terutoshi*; Kimura, Hiroyuki*; Nakao, Akiko*; Ohara, Takashi; Honda, Takashi*; Kodama, Katsuaki; Igawa, Naoki; et al.
Physical Review Research (Internet), 6(1), p.013144_1 - 013144_9, 2024/02
 -tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degree
-tetraoctyldiglycolamide-impregnated polymer-coated silica particle using fluorescence microspectroscopy; Transfer mechanism and effect of polymer crosslinking degreeMiyagawa, Akihisa*; Takahashi, Takumi*; Kuzure, Yoshiaki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Analytical Sciences, 39(11), p.1929 - 1936, 2023/11
Times Cited Count:1 Percentile:10.71(Chemistry, Analytical)In this study, we revealed the Eu(III) distribution in a single diglycolamide-derivative extractant (TODGA)-impregnated polymer-coated silica particle. The reaction of Eu(III) with two TODGA molecules in the polymer layer was the rate-limiting process, as evidenced by the absence of any correlation between the rate constants (k and k
and k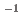 ) and concentrations of Eu(III) and HNO
) and concentrations of Eu(III) and HNO .
.
Watanabe, So; Takahatake, Yoko; Ogi, Hiromichi*; Osugi, Takeshi; Taniguchi, Takumi; Sato, Junya; Arai, Tsuyoshi*; Kajinami, Akihiko*
Journal of Nuclear Materials, 585, p.154610_1 - 154610_6, 2023/11
Times Cited Count:1 Percentile:0.00(Materials Science, Multidisciplinary)Miyagawa, Akihisa*; Hayashi, Naoki*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 96(9), p.1019 - 1025, 2023/09
Times Cited Count:4 Percentile:41.44(Chemistry, Multidisciplinary)In the present study, we have elucidated the mass transfer mechanism of Eu(III) and Sm(III) in the solution with these ions in single nitrilotriacetamide (NTA) extractant-impregnated polymer-coated silica particle. The rate-limiting process of mass transfer was the reaction process of ions with NTA molecules, in which the NO
 ions were not involved, which was consistent with that obtained in single ion distribution system.
ions were not involved, which was consistent with that obtained in single ion distribution system.
Kurihara, Kensuke*; Lobzenko, I.; Tsuru, Tomohito; Serizawa, Ai*
Materials Transactions, 64(8), p.1930 - 1936, 2023/08
Times Cited Count:1 Percentile:11.66(Materials Science, Multidisciplinary)Nanoclusters formed in Al-Mg-Si alloys affect the aging behavior of the alloys depending on the formation temperature. In the present study, first-principles calculations were carried out to evaluate the two- and three-body interactions between Mg, Si atoms and vacancies in the Al matrix and estimate the effect of local bonding on the formation of nanoclusters. Monte Carlo simulations were subsequently performed to investigate the stable structure of the nanocluster formed in Al-0.95 mass pct Mg-0.81 mass pct Si alloy. We found that the Mg-Si and Si-Vac pairs are stable in the Al matrix. The result shows that the solute atoms easily aggregate with different types of solute atoms and that the Si atom has a strong attractive interaction with a vacancy. Furthermore, Mg-Si-vacancy three-body clusters is more stable than Mg-Si and Si-vacancy pairs in the Al matrix. The nanoclusters in the Al matrix were thermally stabilized by the stable configurations between solute atoms and vacancy. Thus, the electronic structure calculations suggested that the local bondings within a nanocluster play a significant role in not only the thermal stability but also the formation and growth behavior of nanoclusters during aging at low temperatures.
Miyagawa, Akihisa*; Hayashi, Naoki*; Kuzure, Yoshiaki*; Takahashi, Takumi*; Iwamoto, Hibiki*; Arai, Tsuyoshi*; Nagatomo, Shigenori*; Miyazaki, Yasunori; Hasegawa, Kenta; Sano, Yuichi; et al.
Bulletin of the Chemical Society of Japan, 96(7), p.671 - 676, 2023/07
Times Cited Count:8 Percentile:61.41(Chemistry, Multidisciplinary)We investigated the distribution mechanism of Eu(III) in a single polymer-coated silica particle including nitrilotriacetamide (NTA) extractants known as HONTA and TOD2EHNTA. The present study provides a valuable approach for the evaluation and enhancement of the functionality of "single extractant-impregnated polymer-coated silica particle".
Akuzawa, Tadashi*; Kim, S.-Y.*; Kubota, Masahiko*; Wu, H.*; Watanabe, So; Sano, Yuichi; Takeuchi, Masayuki; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 331(12), p.5851 - 5858, 2022/12
Times Cited Count:5 Percentile:60.29(Chemistry, Analytical)Collaborative Laboratories for Advanced Decommissioning Science; Shibaura Institute of Technology*
JAEA-Review 2022-008, 116 Pages, 2022/06
The Collaborative Laboratories for Advanced Decommissioning Science (CLADS), Japan Atomic Energy Agency (JAEA), had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project (hereafter referred to "the Project") in FY2020. The Project aims to contribute to solving problems in the nuclear energy field represented by the decommissioning of the Fukushima Daiichi Nuclear Power Station, Tokyo Electric Power Company Holdings, Inc. (TEPCO). For this purpose, intelligence was collected from all over the world, and basic research and human resource development were promoted by closely integrating/collaborating knowledge and experiences in various fields beyond the barrier of conventional organizations and research fields. The sponsor of the Project was moved from the Ministry of Education, Culture, Sports, Science and Technology to JAEA since the newly adopted proposals in FY2018. On this occasion, JAEA constructed a new research system where JAEA-academia collaboration is reinforced and medium-to-long term research/development and human resource development contributing to the decommissioning are stably and consecutively implemented. Among the adopted proposals in FY2018, this report summarizes the research results of the "Development of the sintering solidification method for spent zeolite to long-term stabilization" conducted from FY2018 to FY2021 (this contract was extended to FY2021). Since the final year of this proposal was FY2021, the results for four fiscal years were summarized. The present study aims to develop a new sintering solidification method in which glass is added as a binder to spent zeolite which is adsorbed radionuclides such as Cs and the nuclides are immobilized by sintering them. In this project, the optimum conditions for sintering solidification and the basic performance of the sintered solidified body will be evaluated by cold tests, and they will be demonstrated by hot tests.
Miyagawa, Akihisa*; Takeuchi, Masayuki; Arai, Tsuyoshi*; Watanabe, So; Sano, Yuichi; Nakatani, Kiyoharu*
Bulletin of the Chemical Society of Japan, 95(4), p.566 - 568, 2022/04
Times Cited Count:3 Percentile:20.54(Chemistry, Multidisciplinary)We demonstrate that pKa of extractant impregnated in a polymer phase varies with the cross-linking degree and the coexistence of other extractants, which induces a change in the hydrophobicity of the polymer phase. The results presented herein will be beneficial for the development of novel solid-extraction adsorbents.
Kurihara, Kensuke*; Lobzenko, I.; Tsuru, Tomohito; Serizawa, Ai*
Keikinzoku, 72(2), p.47 - 53, 2022/02
Nanoclusters formed in Al-Mg-Si alloys affect the aging behavior of the alloys depending on the formation temperature. In the present study, first-principles calculations were carried out to evaluate the two- and three-body interactions between Mg, Si atoms and vacancies in the Al matrix and to estimate the effect of local bond structures on the formation of nanoclusters. Monte Carlo simulations were subsequently performed to investigate the stable structure of nanocluster formed in Al-Mg-Si alloy. We found that Mg-Si bond and Si-Vac bond were stable in Al matrix. The result showed that the solute atoms are easy to aggregate with another type of atoms and that Si atom had a strong attractive interaction with a vacancy. Mg-Si-vacancy three-body bond were more stable than Mg-Si two-body bond and Si-vacancy two-body bond in Al matrix. Therefore, vacancies were strongly trapped within the cluster region due to the stable local bonds composed of Mg and Si atoms which indicates that the nanoclusters in Al matrix were thermally stabilized by the stable bonds between solute atoms and vacancy. In addition, these results suggested that inner bonds of nanocluster played a significant role in not only the thermal stability but also the formation and growth behavior of nanoclusters during aging at low temperatures.
Myagmarjav, O.; Tanaka, Nobuyuki; Nomura, Mikihiro*; Noguchi, Hiroki; Imai, Yoshiyuki; Kamiji, Yu; Kubo, Shinji; Takegami, Hiroaki
Progress in Nuclear Energy, 137, p.103772_1 - 103772_7, 2021/07
Times Cited Count:8 Percentile:66.37(Nuclear Science & Technology)Collaborative Laboratories for Advanced Decommissioning Science; Shibaura Institute of Technology*
JAEA-Review 2020-049, 78 Pages, 2021/01
JAEA/CLADS had been conducting the Nuclear Energy Science & Technology and Human Resource Development Project. Among the adopted proposals in FY2018, this report summarizes the research results of the "Development of the Sintering Solidification Method for Spent Zeolite to Long-term Stabilization" conducted in FY2019.
Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nomura, Kazunori; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Hagura, Naoto*; Kubota, Toshio*
Nuclear Instruments and Methods in Physics Research B, 477, p.54 - 59, 2020/08
Times Cited Count:7 Percentile:55.09(Instruments & Instrumentation) RhSi
RhSiYamauchi, Hiroki; Sari, D. P.*; Watanabe, Isao*; Yasui, Yukio*; Chang, L.-J.*; Kondo, Keietsu; Ito, Takashi; Ishikado, Motoyuki*; Hagihara, Masato*; Frontzek, M. D.*; et al.
Communications Materials (Internet), 1, p.43_1 - 43_6, 2020/07
High-temperature short-range order is discovered up to 720 K in Mn RhSi by complementary use of neutron scattering and muon spin relaxation measurements.
RhSi by complementary use of neutron scattering and muon spin relaxation measurements.
Collaborative Laboratories for Advanced Decommissioning Science; Shibaura Institute of Technology*
JAEA-Review 2019-028, 71 Pages, 2020/03
JAEA/CLADS, had been conducting the Center of World Intelligence Project for Nuclear Science/Technology and Human Resource Development (hereafter referred to "the Project") in FY2018. Among the adopted proposals in FY2018, this report summarizes the research results of the "Development of the Sintering Solidification Method for Spent Zeolite to Long-term Stabilization". The present study aims to develop the sintering solidification method for zeolites (spent zeolites) that adsorbs continuously generated radionuclides such as cesium. The sintering solidification method is able to stabilize adsorbed radionuclides such as cesium in zeolites by adding a glass as a binder to spent zeolite and sintered it. It is expected that the sintering solidification method is significantly reduce the volume of the solidified body compare with the glass solidification method and to form a stable solidified body equivalent to the calcination solidification method. In this project, we planned to select a glass suitable for the sintering solidification method and optimize the sintering temperature, etc. using non-radioactive nuclides (cold tests), and verify it by using radioactive nuclides (hot tests). In FY2018, we investigated the thermal properties of candidate glasses for binder and the effect of heating atmosphere on the sintering solidification method. Irradiated fuel for preparing simulated contaminated water containing radionuclides was selected and the condition of it was observed. In addition, we surveyed existing research results and latest research trends about solidification of zeolite, calcination solidification and so on.
Arai, Yoichi; Watanabe, So; Ono, Shimpei; Nomura, Kazunori; Nakamura, Fumiya*; Arai, Tsuyoshi*; Seko, Noriaki*; Hoshina, Hiroyuki*; Kubota, Toshio*
QST-M-23; QST Takasaki Annual Report 2018, P. 59, 2020/03
Watanabe, So; Senzaki, Tatsuya; Shibata, Atsuhiro; Nomura, Kazunori; Takeuchi, Masayuki; Nakatani, Kiyoharu*; Matsuura, Haruaki*; Horiuchi, Yusuke*; Arai, Tsuyoshi*
Journal of Radioanalytical and Nuclear Chemistry, 322(3), p.1273 - 1277, 2019/12
Times Cited Count:6 Percentile:47.17(Chemistry, Analytical)