Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yoshida, Masato; Iguchi, Satoshi; Hirano, Hiroshi*; Kitamura, Akihiro
Nuclear Engineering and Design, 431, p.113691_1 - 113691_16, 2025/01
Times Cited Count:0 Percentile:0.00(Nuclear Science & Technology)The Plutonium Fuel Fabrication Facility is currently in the decommissioning phase, with glovebox dismantling operations ongoing since 2010. During conventional glovebox dismantling operations, the glovebox to be dismantled is enclosed within plastic tents to contain contamination. The glovebox is then dismantled by workers wearing air-fed suits with thermal or mechanical cutting tools, which typically generate dross or sparks in the form of radioactive aerosols during cutting. Despite the longevity and meticulous organization of this manual method, the workload remains considerable, while the allowable working time is limited. In addition, the potential for inhalation exposure to plutonium is elevated in the event of an accident given the contamination of the work area. To overcome disadvantages associated with conventional glovebox dismantling methods, new methods are currently being developed. The primary objective is to reduce the reliance on operation based on air-fed suits and enhance worker safety by introducing remote equipment and a new floor-reinforcing panel. Another objective is to suppress waste generation by reusing all equipment on multiple occasions which is achieved by developing a containment system that have a large open port with a pallet for the storage and reuse of equipment for successive operations. Furthermore, a glove operation compartment is designed and tested for the manual handling of dismantled materials as an additional strategy to reduce work based on air-fed suits and mitigate secondary waste generation.
 Pu
Pu O
O powder using master sintering curve theory
powder using master sintering curve theoryNakamichi, Shinya; Sunaoshi, Takeo*; Hirooka, Shun; Vauchy, R.; Murakami, Tatsutoshi
Journal of Nuclear Materials, 595, p.155072_1 - 155072_11, 2024/07
Times Cited Count:1 Percentile:57.00(Materials Science, Multidisciplinary)Kyffin, J.*; Dia, A.*; Nkosi, G.*; Nizhnik, V.*; Hayashi, Akihiko; Nagatani, Taketeru
Proceedings of 65th Annual Meeting of the Institute of Nuclear Materials Management (Internet), 8 Pages, 2024/07
Kato, Masato; Oki, Takumi; Watanabe, Masashi; Hirooka, Shun; Vauchy, R.; Ozawa, Takayuki; Uwaba, Tomoyuki; Ikusawa, Yoshihisa; Nakamura, Hiroki; Machida, Masahiko
Journal of the American Ceramic Society, 107(5), p.2998 - 3011, 2024/05
Times Cited Count:4 Percentile:34.13(Materials Science, Ceramics)Tsubota, Yoichi; Kimura, Yasuhisa; Nagai, Yuya; Kojima, Sho*; Tokonami, Shinji*; Nakagawa, Takahiro
Proceedings of International Conference on Decommissioning Challenges; Role and importance of innovations (DEM 2024) (Internet), 7 Pages, 2024/05
An in-situ monitoring system for the  -aerosol in the harsh (high humidity, high
-aerosol in the harsh (high humidity, high  /
/ -ray background) environment expected inside the 1F-PCV was developed. A part of the system was installed at the glovebox dismantling site of a MOX fuel facility, and its fast response performance and long-term operation capability were demonstrated.
-ray background) environment expected inside the 1F-PCV was developed. A part of the system was installed at the glovebox dismantling site of a MOX fuel facility, and its fast response performance and long-term operation capability were demonstrated.
Horii, Yuta; Hirooka, Shun; Uno, Hiroki*; Ogasawara, Masahiro*; Tamura, Tetsuya*; Yamada, Tadahisa*; Furusawa, Naoya*; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 588, p.154799_1 - 154799_20, 2024/01
Times Cited Count:7 Percentile:85.99(Materials Science, Multidisciplinary)The thermal conductivities of near-stoichiometric (U,Pu,Am)O doped with Nd
doped with Nd O
O /Sm
/Sm O
O , which is major fission product (FP) generated by a uranium-plutonium mixed oxides (MOX) fuel irradiation, as simulated fission products are evaluated at 1073-1673 K. The thermal conductivities are calculated from the thermal diffusivities that are measured using the laser flash method. To evaluate the thermal conductivity from a homogeneity viewpoint of Nd/Sm cations in MOX, the specimens with different homogeneity of Nd/Sm are prepared using two kinds of powder made by ball-mill and fusion methods. A homogeneous Nd/Sm distribution decreases the thermal conductivity of MOX with increasing Nd/Sm content, whereas heterogeneous Nd/Sm has no influence. The effect of Nd/Sm on the thermal conductivity is studied using the classical phonon transport model (A+BT)
, which is major fission product (FP) generated by a uranium-plutonium mixed oxides (MOX) fuel irradiation, as simulated fission products are evaluated at 1073-1673 K. The thermal conductivities are calculated from the thermal diffusivities that are measured using the laser flash method. To evaluate the thermal conductivity from a homogeneity viewpoint of Nd/Sm cations in MOX, the specimens with different homogeneity of Nd/Sm are prepared using two kinds of powder made by ball-mill and fusion methods. A homogeneous Nd/Sm distribution decreases the thermal conductivity of MOX with increasing Nd/Sm content, whereas heterogeneous Nd/Sm has no influence. The effect of Nd/Sm on the thermal conductivity is studied using the classical phonon transport model (A+BT) . The dependences of the coefficients A and B on the Nd/Sm content (C
. The dependences of the coefficients A and B on the Nd/Sm content (C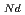 and C
and C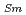 , respectively) are evaluated as: A(mK/W)=1.70
, respectively) are evaluated as: A(mK/W)=1.70  10
10 + 0.93C
+ 0.93C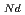 + 1.20C
+ 1.20C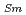 , B(m/W)=2.39
, B(m/W)=2.39  10
10 .
.
Hirooka, Shun; Horii, Yuta; Sunaoshi, Takeo*; Uno, Hiroki*; Yamada, Tadahisa*; Vauchy, R.; Hayashizaki, Kohei; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Science and Technology, 60(11), p.1313 - 1323, 2023/11
Times Cited Count:5 Percentile:80.64(Nuclear Science & Technology)Additive MOX pellets are fabricated by a conventional dry powder metallurgy method. Nd O
O and Sm
and Sm O
O are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
are chosen as the additive materials to simulate the corresponding soluble fission products dispersed in MOX. Shrinkage curves of the MOX pellets are obtained by dilatometry, which reveal that the sintering temperature is shifted toward a value higher than that of the respective regular MOX. The additives, however, promote grain growth and densification, which can be explained by the effect of oxidized uranium cations covering to a pentavalent state. Ceramography reveals large agglomerates after sintering, and Electron Probe Micro-Analysis confirms that inhomogeneous elemental distribution, whereas XRD reveals a single face-centered cubic phase. Finally, by grinding and re-sintering the specimens, the cation distribution homogeneity is significantly improved, which can simulate spent nuclear fuels with soluble fission products.
Kitamura, Akihiro; Hirano, Hiroshi*; Yoshida, Masato
Nuclear Engineering and Design, 411, p.112435_1 - 112435_14, 2023/09
Times Cited Count:1 Percentile:25.62(Nuclear Science & Technology)This study presents the features and brief history of the glovebox dismantling facility and the primary dismantlement results. Subsequently, we evaluate the novelties of the facility from operational experiences in manual and remote glovebox dismantlement methods and discuss their characteristics. Furthermore, we evaluate the worker exposure dose based on the obtained data. Finally, we show how these experiences are effectively fed back to the technological dismantlement development for our decommissioning project.
Shibanuma, Tomohiro; Hirano, Hiroshi*; Kimura, Yasuhisa; Aita, Takahiro; Yoshida, Masato; Nagai, Yuya; Kitamura, Akihiro
Hoken Butsuri (Internet), 58(2), p.91 - 98, 2023/08
We developed new containment tents that are more easily assembled and effectively functioned, by improving and refurbishing the shortcomings of the conventional tents. The new tents have been already tested in the real airborne contamination situation occurred at the plutonium fuel fabricating facility. The tents appropriately functioned for intended use but other shortcomings emerged and therefore we had modified the structure of the tents further.
Kitamura, Akihiro; Hirano, Hiroshi*; Yoshida, Masato; Takeuchi, Kentaro
Hoken Butsuri (Internet), 58(2), p.76 - 90, 2023/08
The alpha contaminated gloveboxes have been dismantled for over 20 years in Plutonium Fuel Fabrication Facility. The so called wet recovery equipment gloveboxes, which recover plutonium and uranium from scrap fuel by dissolving and extracting processes, were chosen as the priority gloveboxes to be dismantled. These gloveboxes and other gloveboxes in the same room were size reduced and removed up until 2022. Also, non-radioactive ancillary facility and non-radioactive giant glovebox were removed from 2007 to 2010 for ease of glovebox dismantling activities that follows and for making waste storage spaces. Several incidents were occurred and recidivism prevention measures were implemented on each occasion. In this report, glovebox dismantling activities we conducted in the past 20 years are reviewed and lessons we have learned are summarized.
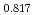 Pu
Pu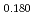 Am
Am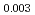 O
O uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 K
uranium-plutonium-americium mixed oxides at 1573, 1773, and 1873 KVauchy, R.; Sunaoshi, Takeo*; Hirooka, Shun; Nakamichi, Shinya; Murakami, Tatsutoshi; Kato, Masato
Journal of Nuclear Materials, 580, p.154416_1 - 154416_11, 2023/07
Times Cited Count:9 Percentile:94.10(Materials Science, Multidisciplinary)Kato, Masato; Nakamichi, Shinya; Hirooka, Shun; Watanabe, Masashi; Murakami, Tatsutoshi; Ishii, Katsunori
Nihon Genshiryoku Gakkai Wabun Rombunshi (Internet), 22(2), p.51 - 58, 2023/04
Uranium and Plutonium mixed oxide (MOX) pellets used as fast reactor fuels have been produced from several raw materials by mechanical blending method through processes of ball milling, additive blending, granulation, pressing, sintering and so on. It is essential to control the pellet density which is one of the important fuel specifications, but it is difficult to understand relationships among many parameters in the production. Database for MOX production was prepared from production results in Japan, and input data of eighteen types were chosen from production process and made a data set. Machine learning model to predict sintered density of MOX pellet was derived by gradient boosting regressor, and represented the measured sintered density with coefficient of determination of R =0.996
=0.996
Kageyama, Tomio; Denuma, Akio; Koizumi, Jin*; Odakura, Manabu*; Haginoya, Masahiro*; Isaka, Shinichi*; Kadowaki, Hiroyuki*; Kobayashi, Shingo*; Morimoto, Taisei*; Kato, Yoshiaki*; et al.
JAEA-Technology 2022-033, 130 Pages, 2023/03
Uranium handling facility for development of nuclear fuel manufacturing equipment (Mockup room) was constructed in 1972. The Mockup room has a weak seismic resistance and is deteriorating with age. Also, the original purpose with this facility have been achieved and there are no new development plans using this facility. Therefore, interior equipment installed in this facility had been dismantled and removed since March 2019. After that, the Mockup room was inspected for contamination, and then controlled area in the Mockup room was cancelled on March 29th 2022. A total of 6,549 workers (not including security witnesses) were required for this work. The amount of non-radioactive waste generated by this work was 31,300 kg. The amount of radioactive waste generated by this work was 3,734 kg of combustible waste (103 drums), 4,393 kg of flame resistance waste (61 drums), 37,790 kg of non-combustible waste (124 drums, 19 containers). This report describes about the dismantling and removing the interior equipment in the Mockup room, the amount of waste generated by this work, and procedure for cancellation the controlled area in the facility.
Kawasaki, Kohei; Ono, Takanori; Shibanuma, Kimikazu; Goto, Kenta; Aita, Takahiro; Okamoto, Naritoshi; Shinada, Kenta; Ichige, Hidekazu; Takase, Tatsuya; Osaka, Yuki; et al.
JAEA-Technology 2022-031, 91 Pages, 2023/02
The document for back-end policy opened to the public in 2018 by Japan Atomic Energy Agency (hereafter, JAEA) states the decommissioning of facilities of Nuclear Fuel Cycle Engineering Laboratories and JAEA have started gathering up nuclear fuel material of the facilities into Plutonium Fuel Production Facilities (hereafter, PFPF) in order to put it long-term, stable and safe storage. Because we planned to manufacture scrap assemblies almost same with Monju fuel assembly using unsealed plutonium-uranium mixed-oxide (hereafter, MOX) powder held in PFPF and transfer them to storage facilities as part of this "concentration" task of nuclear fuel material, we obtained permission to change the use of nuclear fuel material in response to the new regulatory Requirements in Japan for that. The amount of plutonium (which is neither sintered pellets nor in a lidded powder-transport container) that could be handled in the pellet-manufacturing process was limited to 50 kg Pu or less in order to decrease the facility risk in this manufacture. Therefore, we developed and installed the "MOX weighing and blending equipment" corresponding with small batch sizes that functioned in a starting process and the equipment would decrease handling amounts of plutonium on its downstream processes. The failure data based on our operation and maintenance experiences of MOX fuel production facilities was reflected in the design of the equipment to further improve reliability and maintainability in this development. The completed equipment started its operation using MOX powder in February 2022 and the design has been validated through this half-a-year operation. This report organizes the knowledge obtained through the development of the equipment, the evaluation of the design based on the half-a-year operation results and the issues in future equipment development.
 ceramics
ceramicsVauchy, R.; Hirooka, Shun; Watanabe, Masashi; Yokoyama, Keisuke; Sunaoshi, Takeo*; Yamada, Tadahisa*; Nakamichi, Shinya; Murakami, Tatsutoshi
Ceramics International, 49(2), p.3058 - 3065, 2023/01
Times Cited Count:11 Percentile:64.70(Materials Science, Ceramics)
Watanabe, Masashi; Kato, Masato
Frontiers in Nuclear Engineering (Internet), 1, p.1082324_1 - 1082324_9, 2023/01
Since the oxygen potential and the oxygen coefficient of UO have a significant impact on fuel performance, many experimental data have been obtained. However, experimental data of the oxygen potential and the oxygen diffusion coefficient in the high temperature region above 1673 K are very limited. In the present study, we aimed to obtain these data and analyze them by defect chemistry. The oxygen potentials and the oxygen chemical diffusion coefficient of UO
have a significant impact on fuel performance, many experimental data have been obtained. However, experimental data of the oxygen potential and the oxygen diffusion coefficient in the high temperature region above 1673 K are very limited. In the present study, we aimed to obtain these data and analyze them by defect chemistry. The oxygen potentials and the oxygen chemical diffusion coefficient of UO were measured by the gas equilibrium method in the near stoichiometric region at temperatures ranging from 1673 to 1873 K. A data set of oxygen potentials was made together with literature data and analyzed by defect chemistry. The oxygen potential of UO
were measured by the gas equilibrium method in the near stoichiometric region at temperatures ranging from 1673 to 1873 K. A data set of oxygen potentials was made together with literature data and analyzed by defect chemistry. The oxygen potential of UO was determined as a function of O/U ratio and temperature, and an equation representing the relationship was derived. The oxygen chemical diffusion coefficient values obtained in this study were reasonably close to the literature values. The oxygen partial pressure dependence of the oxygen chemical diffusion coefficients was predicted from the evaluated results of the oxygen potential data, but no clear dependence was observed.
was determined as a function of O/U ratio and temperature, and an equation representing the relationship was derived. The oxygen chemical diffusion coefficient values obtained in this study were reasonably close to the literature values. The oxygen partial pressure dependence of the oxygen chemical diffusion coefficients was predicted from the evaluated results of the oxygen potential data, but no clear dependence was observed.
 , ThO
, ThO , UO
, UO , PuO
, PuO , and (U, Pu)O
, and (U, Pu)O
Kato, Masato; Watanabe, Masashi; Hirooka, Shun; Vauchy, R.
Frontiers in Nuclear Engineering (Internet), 1, p.1081473_1 - 1081473_10, 2023/01
Kato, Masato; Machida, Masahiko; Hirooka, Shun; Nakamichi, Shinya; Ikusawa, Yoshihisa; Nakamura, Hiroki; Kobayashi, Keita; Ozawa, Takayuki; Maeda, Koji; Sasaki, Shinji; et al.
Materials Science and Fuel Technologies of Uranium and Plutonium mixed Oxide, 171 Pages, 2022/10
Innovative and advanced nuclear reactors using plutonium fuel has been developed in each country. In order to develop a new nuclear fuel, irradiation tests are indispensable, and it is necessary to demonstrate the performance and safety of nuclear fuels. If we can develop a technology that accurately simulates irradiation behavior as a technology that complements the irradiation test, the cost, time, and labor involved in nuclear fuel research and development will be greatly reduced. And safety and reliability can be significantly improved through simulation of nuclear fuel irradiation behavior. In order to evaluate the performance of nuclear fuel, it is necessary to know the physical and chemical properties of the fuel at high temperatures. And it is indispensable to develop a behavior model that describes various phenomena that occur during irradiation. In previous research and development, empirical methods with fitting parameters have been used in many parts of model development. However, empirical techniques can give very different results in areas where there is no data. Therefore, the purpose of this study is to construct a scientific descriptive model that can extrapolate the basic characteristics of fuel to the composition and temperature, and to develop an irradiation behavior analysis code to which the model is applied.
Kawaguchi, Koichi; Segawa, Tomoomi; Ishii, Katsunori
Funtai Kogakkai-Shi, 59(6), p.283 - 290, 2022/06
In the Japan Atomic Energy Agency, in order to effectively use the out-of-standard pellets in the fuel manufacturing process for high-speed furnaces, we are developing techniques for crushing and reusing them with raw material powder. By analyzing in detail the particle size distribution before and after grinding, it was shown that the grinding powder is composed of three different component particles having different characteristics of the particle size distribution. In addition, we examined the method of predicting pulverized powder particle size distribution from the supply powder particle size distribution.
Hirooka, Shun; Yokoyama, Keisuke; Kato, Masato
Proceedings of International Conference on Fast Reactors and Related Fuel Cycles; Sustainable Clean Energy for the Future (FR22) (Internet), 8 Pages, 2022/04
Property studies on Am/Np-bearing MOX were carried out and how the properties influences on the irradiation behaviors was discussed. Both Am and Np inclusions increase the oxygen potential of MOX. Inter-diffusion coefficients obtained by using diffusion couple technique indicate that the inter-diffusion coefficient is larger in the order of U-Am, U-Pu and U-Np. Also, the inter-diffusion coefficients were evaluated to be larger at the O/M = 2 than those of O/M  2 by several orders. The increase of oxygen potential with Am/Np leads to higher vapor pressure of UO
2 by several orders. The increase of oxygen potential with Am/Np leads to higher vapor pressure of UO and the acceleration of the pore migration along temperature gradient during irradiation. The redistributions of actinide elements were also considered with the relationship of the pore migration and diffusion in solid state. Thus, the obtained inter-diffusion coefficients directly influence on the redistribution rate. The obtained properties were modelled and can be installed in a fuel irradiation simulation code.
and the acceleration of the pore migration along temperature gradient during irradiation. The redistributions of actinide elements were also considered with the relationship of the pore migration and diffusion in solid state. Thus, the obtained inter-diffusion coefficients directly influence on the redistribution rate. The obtained properties were modelled and can be installed in a fuel irradiation simulation code.