Physical and chemical properties of sodium ferrates and equilibrium calculations in H O/CO
O/CO environments
environments
Huang, J.; Furukawa, Tomohiro 
 ; Aoto, Kazumi
; Aoto, Kazumi 
Thermodynamic data for most of the Na-Fe oxides with Fe+2 and Fe+3 have been evaluated in previous studies by the present authors; how- ever, crystal structureand thermodynamic data Na4feO11 (or Na2O1.5Fe2O3) have seldom been studied experimentally. To investigate its role in the Na-Fe-O system, sample Na4Fe6O11 was prepared by heating 2Na2CO3+3Fe2O3. The X-ray powder diffraction data of Nqa4Fe6O11 were indexed by a monoclinic system with the cell parameters a = 13.4622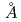 , b = 5.3886
, b = 5.3886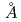 , c = 9.1317
, c = 9.1317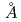 ,
,  = 90
= 90 ,
,  = 96.35
= 96.35 ,
,  = 90.0
= 90.0 . By using Knudsen effu-sion mass spectrometry, the temperature dependence of CO2 partial pre-ssure overthe mixture 2Na2CO3+3Fe2O3 was determined as ln{p(CO2)/Pa}=29.4385-29015.4/T(913-1023K). Thermodynamic data of Na4Fe6O11 were then evaluated as,
. By using Knudsen effu-sion mass spectrometry, the temperature dependence of CO2 partial pre-ssure overthe mixture 2Na2CO3+3Fe2O3 was determined as ln{p(CO2)/Pa}=29.4385-29015.4/T(913-1023K). Thermodynamic data of Na4Fe6O11 were then evaluated as,  fH
fH (298.15 K) = -3569.54
(298.15 K) = -3569.54 3.95 KJ/mol = (-3716839
3.95 KJ/mol = (-3716839 2274.55) + (1200.16
2274.55) + (1200.16 2.35)
2.35)  T/K,
T/K,  fG
fG (298) is recommended as -3255.3
(298) is recommended as -3255.3 12.4 KJ/mol. It is further found that Na4fe6O11is thermodynamically stable only at high temperatures over about 1200K
12.4 KJ/mol. It is further found that Na4fe6O11is thermodynamically stable only at high temperatures over about 1200K