Neptunium carbonato complexes in aqueous solution; An Electrochemical, spectroscopic, and quantum chemical study
水溶液中のネプツニウム炭酸錯体に関する電気化学,分光学、及び量子化学的研究
池田 篤史 
 ; 津島 悟*; 鷹尾 康一朗*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; 矢板 毅; Hennig, C.*
; 津島 悟*; 鷹尾 康一朗*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; 矢板 毅; Hennig, C.*
Ikeda, Atsushi; Tsushima, Satoru*; Takao, Koichiro*; Rossberg, A.*; Funke, H.*; Scheinost, A. C.*; Bernhard, G.*; Yaita, Tsuyoshi; Hennig, C.*
水溶液中で形成されるネプツニウム(Np)の炭酸錯体について、電気化学的手法,紫外可視・赤外分光法,X線吸収分光法、及び量子化学計算を用いて、その電気化学的及び錯体化学的特性を検討した。その結果、V価及びVI価のNpは1.5M Na CO
CO 溶液中においてネプツニル三炭酸錯体である[NpO
溶液中においてネプツニル三炭酸錯体である[NpO (CO
(CO )
) ]
]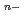 を形成していること、さらにpH=13以上の2.0M Na
を形成していること、さらにpH=13以上の2.0M Na CO
CO /1.0M NaOH溶液中ではNp(V)は電気的にNp(VII)まで酸化され、劇的な錯体構造の変化を伴い[NpO
/1.0M NaOH溶液中ではNp(V)は電気的にNp(VII)まで酸化され、劇的な錯体構造の変化を伴い[NpO (OH)
(OH) ]
] を形成していること等が明らかとなった。
を形成していること等が明らかとなった。
The electrochemical behavior and complex structure of Np carbonato complexes have been investigated in aqueous Na CO
CO and Na
and Na CO
CO /NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na
/NaOH solutions at different oxidation states by using cyclic voltammetry, X-ray absorption spectroscopy, and density functional theory calculations. The end-member complexes of penta- and hexavalent Np in 1.5 M Na CO
CO with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO
with pH = 11.7 have been determined as a transdioxo neptunyl tricarbonato complex, [NpO (CO
(CO )
) ]
]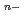 (
( = 5 for Np
= 5 for Np , and 4 for Np
, and 4 for Np ). In contrast, the electrochemical oxidation of Np
). In contrast, the electrochemical oxidation of Np in a highly basic carbonate solution of 2.0M Na
in a highly basic carbonate solution of 2.0M Na CO
CO /1.0M NaOH (pH
/1.0M NaOH (pH  13) yielded a stable heptavalent Np complex of [Np
13) yielded a stable heptavalent Np complex of [Np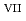 O
O (OH)
(OH) ]
] , indicating that the oxidation reaction from Np
, indicating that the oxidation reaction from Np to Np
to Np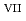 in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
in the carbonate solution involves a drastic structural rearrangement from the transdioxo configuration to a square-planar-tetraoxo configuration, as well as exchanging the coordinating anions from carbonate ions (CO
 ) to hydroxide ions (OH
) to hydroxide ions (OH ).
).