Pulse radiolysis study on free radical scavenger edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), 2; A Comparative study on edaravone derivatives
フリーラジカル捕捉剤エダラボン(3-methyl-1-phenyl-2-pyrazolin-5-one)のパルスラジオリシス実験,2; エダラボン誘導体を用いた比較研究
端 邦樹  ; Lin, M.; 勝村 庸介*; 室屋 裕佐*; Fu, H. Y.*; 山下 真一; 中川 秀彦*
; Lin, M.; 勝村 庸介*; 室屋 裕佐*; Fu, H. Y.*; 山下 真一; 中川 秀彦*
Hata, Kuniki; Lin, M.; Katsumura, Yosuke*; Muroya, Yusa*; Fu, H. Y.*; Yamashita, Shinichi; Nakagawa, Hidehiko*
A comparative study using the pulse radiolysis technique was carried out to investigate transient absorption spectra and rate constants for the reactions of OH radical and N radical with edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) and its four analogue compounds, 1,3-dimethyl-2-pyrazolin- 5-one, 3-methyl-1-(pyridin-2-yl)-2-pyrazolin-5-one, 1-phenyl-3-trifluoromethyl-2-pyrazolin-5-one and 1-(4-chlorophenyl)-3-ethyl-2-pyrazolin-5-one. The results showed that, unlike reaction mechanisms previously proposed, the phenyl group of edaravone played an important role in the reaction with OH radical and OH adducts to the phenyl group were formed. Quantum chemical calculations also strongly supported this attribution and suggested that the most favorable site for attacks by OH radical is the ortho position of the phenyl group. Moreover, the rate constants for the reactions of edaravone and its analogues towards OH radical and N
radical with edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one) and its four analogue compounds, 1,3-dimethyl-2-pyrazolin- 5-one, 3-methyl-1-(pyridin-2-yl)-2-pyrazolin-5-one, 1-phenyl-3-trifluoromethyl-2-pyrazolin-5-one and 1-(4-chlorophenyl)-3-ethyl-2-pyrazolin-5-one. The results showed that, unlike reaction mechanisms previously proposed, the phenyl group of edaravone played an important role in the reaction with OH radical and OH adducts to the phenyl group were formed. Quantum chemical calculations also strongly supported this attribution and suggested that the most favorable site for attacks by OH radical is the ortho position of the phenyl group. Moreover, the rate constants for the reactions of edaravone and its analogues towards OH radical and N radical were about 8.0
radical were about 8.0 10
10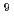 , and 4.0
, and 4.0 10
10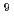 dm
dm mol
mol s
s , respectively. Edaravone displayed higher reactivity compared to the others, in contrast a previous report in which 3-methyl-1-(pyridin-2-yl)-2-pyrazolin-5-one showed the highest reactivity towards OH radical.
, respectively. Edaravone displayed higher reactivity compared to the others, in contrast a previous report in which 3-methyl-1-(pyridin-2-yl)-2-pyrazolin-5-one showed the highest reactivity towards OH radical.