Electrochemical characteristics of layered Li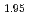 Mn
Mn Co
Co O
O (
(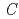 2/
2/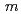 ) as a lithium-battery cathode
) as a lithium-battery cathode
リチウムイオン2次電池用正極材・層状Li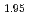 Mn
Mn Co
Co O
O の電気化学的特性
の電気化学的特性
小澤 清*; 中尾 靖宏*; 茂筑 高士*; Cheng, Z.*; Wang, L.*; 岩井 秀夫*; 土屋 佳則*; 藤井 宏樹*; 井川 直樹 


Ozawa, Kiyoshi*; Nakao, Yasuhiro*; Mochiku, Takashi*; Cheng, Z.*; Wang, L.*; Iwai, Hideo*; Tsuchiya, Yoshinori*; Fujii, Hiroki*; Igawa, Naoki
リチウムイオン2次電池正極材Li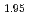 Mn
Mn Co
Co O
O を共沈法によって作製し、その電気化学的特性を解析した。中性子回折実験及びRietveld解析によって求めた本材料の結晶構造は、空間群
を共沈法によって作製し、その電気化学的特性を解析した。中性子回折実験及びRietveld解析によって求めた本材料の結晶構造は、空間群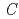 2/
2/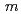 のLi
のLi MnO
MnO 型と同定できた。本材料の充放電サイクル特性は、電流密度が30mAhg
型と同定できた。本材料の充放電サイクル特性は、電流密度が30mAhg の場合は、2.0-4.8Vの電圧域で最初からの11サイクルまでに放電能が46.3から196.5mAhg
の場合は、2.0-4.8Vの電圧域で最初からの11サイクルまでに放電能が46.3から196.5mAhg へと増加し、その後23から58サイクルでは175.5mAhg
へと増加し、その後23から58サイクルでは175.5mAhg を保持していた。サイクリックボルタモグラム及びX線光電子分光実験の結果から、本材料では最初の10サイクル程度でMnの還元反応が活性化されていることが明らかとなった。
を保持していた。サイクリックボルタモグラム及びX線光電子分光実験の結果から、本材料では最初の10サイクル程度でMnの還元反応が活性化されていることが明らかとなった。
A manganese-based solid solution with the composition of Li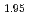 Mn
Mn Co
Co O
O was synthesized by a simplified coprecipitation method, and its electrochemical characteristics as a lithium-battery cathode were investigated. Rietveld refinement based on neutron diffraction data revealed that the material is assigned to an Li
was synthesized by a simplified coprecipitation method, and its electrochemical characteristics as a lithium-battery cathode were investigated. Rietveld refinement based on neutron diffraction data revealed that the material is assigned to an Li MnO
MnO -type structure model with a space group symmetry of
-type structure model with a space group symmetry of 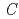 2/
2/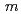 . In cycling of the cell in the potential range from 2.0 to 4.8 V at current densities of 30 mAhg
. In cycling of the cell in the potential range from 2.0 to 4.8 V at current densities of 30 mAhg , the discharge capacity characteristically increases from 46.3 to 196.5 mAhg
, the discharge capacity characteristically increases from 46.3 to 196.5 mAhg as the cycle increases from 1 to 11, and a discharge capacity above 175.5 mAhg
as the cycle increases from 1 to 11, and a discharge capacity above 175.5 mAhg is obtained between the 23rd and 58th cycles. The cyclic voltammogram and X-ray photoelectron spectroscopy measurements showed that the manganese redox reaction is progressively activated during the first ten-odd cycles.
is obtained between the 23rd and 58th cycles. The cyclic voltammogram and X-ray photoelectron spectroscopy measurements showed that the manganese redox reaction is progressively activated during the first ten-odd cycles.