Molar entropy of the selenium (VI)/(IV) couple obtained by cyclic voltammetry
Doi, Reisuke 

Cyclic voltammetry was performed to determine the molar entropy of the Se(VI/IV) couple. In order to obtain the ion interaction coefficient between HSeO
 and Na
and Na ,
, 
 (HSeO
(HSeO
 , Na
, Na ), the following reaction involving uncharged species as reductant was used: HSeO
), the following reaction involving uncharged species as reductant was used: HSeO
 + 3H
+ 3H + 2e
+ 2e = H
= H SeO
SeO + H
+ H O. Half wave potentials were measured in acidic sodium nitrate solutions as a function of the molality of Na
O. Half wave potentials were measured in acidic sodium nitrate solutions as a function of the molality of Na . A number of temperatures were used: 15, 25, 35 and 50
. A number of temperatures were used: 15, 25, 35 and 50 C. SIT was used to calculate the HSeO
C. SIT was used to calculate the HSeO
 /H
/H SeO
SeO standard potential,
standard potential, 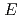
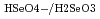 (
( , 0), and
, 0), and 
 (HSeO
(HSeO
 , Na
, Na ) at each temperature. The following molar entropy was derived from the temperature dependence of
) at each temperature. The following molar entropy was derived from the temperature dependence of 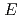
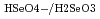 (
( , 0):
, 0): 
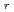 S
S
 /2
/2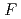 = -0.3
= -0.3 0.3 mV K
0.3 mV K . At 25
. At 25 C
C 
 (HSeO
(HSeO
 , Na
, Na ) was 0.29
) was 0.29 0.04 kg mole
0.04 kg mole .
.