Thermodynamic properties and revised Helgeson-Kirkham-Flowers equations of state parameters of the hydrated and dehydrated monomeric silica species at  = 0.01-600
= 0.01-600 C,
C,  = 1-3000 bars,
= 1-3000 bars, 
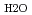 = 0.35-1.1 g cm
= 0.35-1.1 g cm , and
, and 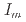 = 0
= 0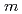
Walker, C. S.*; Arthur, R. C.*; Anraku, Sohtaro  ; Sasamoto, Hiroshi
; Sasamoto, Hiroshi 

 ; Mihara, Morihiro
; Mihara, Morihiro 

The thermodynamic properties and revised Helgeson-Kirkham-Flowers equation of state (r-H-K-F EoS) parameters of the hydrated (Si(OH) (aq), SiO(OH)
(aq), SiO(OH)
 and SiO
and SiO (OH)
(OH)
 ) and fictive dehydrated (SiO
) and fictive dehydrated (SiO (aq), HSiO
(aq), HSiO
 and SiO
and SiO
 ) monomeric silicon species are used extensively to describe the pH, composition, temperature, and pressure dependence of formation/breakdown reactions of all silicon-bearing compounds globally. Experimental log10 equilbrium constant, K values describing the formation reactions of the hydrated and dehydrated monomeric silicon species were therefore compiled from the literature, extrapolated to zero ionic strength by specific ion interaction theory as required and used to derive their thermodynamic properties and r-H-K-F EoS parameters. Consideration of all formation reactions in the same study provides a collective, internally consistent update to the thermodynamic properties and r-H-K-F EoS parameters of the monomeric silicon species that can provide a satisfactory match to the experimental log10 K values at
) monomeric silicon species are used extensively to describe the pH, composition, temperature, and pressure dependence of formation/breakdown reactions of all silicon-bearing compounds globally. Experimental log10 equilbrium constant, K values describing the formation reactions of the hydrated and dehydrated monomeric silicon species were therefore compiled from the literature, extrapolated to zero ionic strength by specific ion interaction theory as required and used to derive their thermodynamic properties and r-H-K-F EoS parameters. Consideration of all formation reactions in the same study provides a collective, internally consistent update to the thermodynamic properties and r-H-K-F EoS parameters of the monomeric silicon species that can provide a satisfactory match to the experimental log10 K values at  = 0.01-600
= 0.01-600 C,
C,  = 1-3000 bars,
= 1-3000 bars, 
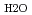 = 0.35-1.1 g cm
= 0.35-1.1 g cm , and zero ionic strength. These temperature and pressure limits comfortably bracket t=0.01-100
, and zero ionic strength. These temperature and pressure limits comfortably bracket t=0.01-100 C and P =1-270 bars relecant to the geological disposal of radioactive wastes at depths of up to 1 km.
C and P =1-270 bars relecant to the geological disposal of radioactive wastes at depths of up to 1 km.