Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 , I, I
, I, I , and CsI on C
, and CsI on C fullerene
fullereneKobayashi, Takanori; Yokoyama, Keiichi
Journal of Nuclear Science and Technology, 53(10), p.1489 - 1493, 2016/10
Times Cited Count:6 Percentile:44.70(Nuclear Science & Technology)Theoretical investigation for the adsorption of the cesium atom (Cs), the cesium iodide molecule (CsI), the iodine atom (I), the cesium cation (Cs ), and the iodide anion (I
), and the iodide anion (I ) onto the surface of a single fullerene molecule (C
) onto the surface of a single fullerene molecule (C ) are reported. A hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP) is employed. The adsorption energies, i.e., the opposite of enthalpy change through adsorption, are calculated to be 34, 3, 2, 11, and 12 kcal mol
) are reported. A hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP) is employed. The adsorption energies, i.e., the opposite of enthalpy change through adsorption, are calculated to be 34, 3, 2, 11, and 12 kcal mol for Cs, CsI, I, Cs
for Cs, CsI, I, Cs , and I
, and I , respectively. The equilibrium constant for Cs is calculated to be 7
, respectively. The equilibrium constant for Cs is calculated to be 7 10
10 atm
atm at the temperature of 1000 K and is seven orders of magnitude higher than that for CsI, indicating that the C
at the temperature of 1000 K and is seven orders of magnitude higher than that for CsI, indicating that the C molecule adsorb the Cs atom highly selectively against the CsI molecule.
molecule adsorb the Cs atom highly selectively against the CsI molecule.
Yamaguchi, Tetsuji
JAERI-Data/Code 2000-031, 131 Pages, 2000/11
no abstracts in English
Savage, D.*; Lemke, K.*; Sasamoto, Hiroshi; Shibata, Masahiro; Arthur, R. C,*; Yui, Mikazu
JNC TN8400 2000-004, 30 Pages, 2000/01
Modeling approaches that have been proposed for cement-water system are reviewed in this report, and relevant supporting thsrmodynamic data are compiled. The thermodynamic data include standard molal thermodynamic properties of minerals and related compounds comprising cements, and equilibrium constants for associated hydrolysis reactions. Similar data for minerals that are stable in hyperalkaline geologic environments (e.g., zeolites) are also included because these minerals could be formed as hyperalkaline fluids emanating from cementitious matelials in a repository for radioactive wastes interact with the surrounding host rock. Standard molal properties (i.e., standard molal Gibbs free energies and enthalpies of formation and standard molal entropies), and/or equilibrium constants for associated hydrolysis reactions, are included for. (1)cement minerals and related compounds (Reardon, 1992; Glasser et al., 1999) (2)calcium-silicate hydrate minerals (Sarkar et al., 1982), and (3)zeolites (calorimetric and estimated values from various sources) All these data are accepted at face value, and it is therefore cautioned that the data, considered as a whole, may not be internally consistent. It is also important to note that the accuracy of these data have not been evaluated in the present study. Several models appropriate for cement-water systems have been proposed in recent years. Most are similar in the sense that they represent empirical fits to laboratory data for the CSH gel-water system, and therefore not thermodynamically defensible. An alternative modeling approach based on thermodynamic principles of solid-solution behavior appropriate for CSH gel has recently been proposed, however. It is reviewed in the present study, and evaluated in relation to experimental results obtained by JNC on cement-water interactions. The solid-solution model is based upon a thermodynamically- and structually-justifiable description of CSH gel in terms of a non-ideal ...
Yui, Mikazu; ; Shibata, Masahiro
JNC TN8400 99-070, 106 Pages, 1999/11
This report is a summary of status, frozen datasets, and future tasks of the JNC thermodynamic database (JNC-TDB) for assessing performance of high-level radioactive waste in geological environments. The JNC-TDB development was carried out after the first progress report on geological disposal research in Japan (H3). In the development, thermodynamic data (equilibrium constants at 25  C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
C, I=0) for important radioactive elements were selected/determined based on original experimental data using different models (e.g., SIT, Pitzer). As a result, the reliability and traceability of the data for most of the important elements were improved over those of the PNC-TDB used in H-3 report. For detailed information of data analysis and selections for each element, see the JNC technical reports listed in this document.
Oda, Chie; Arthur, R. C,*; Sasamoto, Hiroshi; Shibata, Masahiro; Yui, Mikazu; Neyama, Atsushi*
JNC TN8400 99-079, 287 Pages, 1999/09
Two thermodynamic databases for geochemical calculations supporting research and development on geological disposal concepts for high level radioactive waste are described in this report. One, SPRONS.JNC, is compatible with thermodynamic relations comprising the SUPCRT model and software, which permits calculation of the standard molal and partial molal thermodynamic properties of minerals, gases, aqueous species and reactions from 1 to 5000 bars and 0 to 1000 C. This database includes standard molal Gibbs free energies and enthalpies of formation, standard molal entropies and volumes, and Maier-Kelly heat capacity coefficients at the reference pressure (1 bar) and temperature (25
C. This database includes standard molal Gibbs free energies and enthalpies of formation, standard molal entropies and volumes, and Maier-Kelly heat capacity coefficients at the reference pressure (1 bar) and temperature (25 C) for 195 minerals and 16 gases. It also includes standard partial molal Gibbs free energies and enthalpies of formation, standard partial molal entropies, and Helgeson, Kirkham and Flowers (HKF) equation-of-state coefficients at the reference pressure and temperature for 1147 inorganic and organic aqueous ions and complexes. SPRONS.JNC extends similar databases described elsewhere by incorporating new and revised data published in the peer-reviewed literature since 1991. The other database, PHREEQE.JNC, is compatible with the PHREEQE series of geochemical modeling codes. It includes equilibrium constants at 25
C) for 195 minerals and 16 gases. It also includes standard partial molal Gibbs free energies and enthalpies of formation, standard partial molal entropies, and Helgeson, Kirkham and Flowers (HKF) equation-of-state coefficients at the reference pressure and temperature for 1147 inorganic and organic aqueous ions and complexes. SPRONS.JNC extends similar databases described elsewhere by incorporating new and revised data published in the peer-reviewed literature since 1991. The other database, PHREEQE.JNC, is compatible with the PHREEQE series of geochemical modeling codes. It includes equilibrium constants at 25 C and 1 bar for mineral-dissolution, gas-solubility, aqueous-association and oxidation-reduction reactions. Reaction enthalpies, or coefficients in an empirical log K(T) function, are also included in this database, which permits calculation of equilibrium constants between 0 and 100
C and 1 bar for mineral-dissolution, gas-solubility, aqueous-association and oxidation-reduction reactions. Reaction enthalpies, or coefficients in an empirical log K(T) function, are also included in this database, which permits calculation of equilibrium constants between 0 and 100 C at 1 bar. All equilibrium constants, reaction enthalpies, and logK(T) coefficients in PHREEQE.JNC are calculated usig SUPCRT and SPRONS.JNC, which ensures that these two databases are mutually consistent. They are also internally consistent insofar as all the data are compatible with basic thermodynamic definitions and functional relations in the SUPCRT ...
C at 1 bar. All equilibrium constants, reaction enthalpies, and logK(T) coefficients in PHREEQE.JNC are calculated usig SUPCRT and SPRONS.JNC, which ensures that these two databases are mutually consistent. They are also internally consistent insofar as all the data are compatible with basic thermodynamic definitions and functional relations in the SUPCRT ...

 and AnO
and AnO
 Species in Geologic Environments
Species in Geologic EnvironmentsChoppin, G. R.*; Bronikowski, M.*; Chen, J.*; Byegard, J.*; Rai, D.*; Yui, Mikazu
JNC TN8400 99-012, 155 Pages, 1999/01
This report provides thermodynamic data for predicting concentrations of pentavalent and hexavalent actinide species (AnO and AnO
and AnO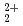 ) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO
) in geologic environments, and contributes to an integration of the JNC chemical thermodynamic database, JNC-TDB (previously PNC-TDB), for the performance analysis of geological isolation system for high-level radioactive wastes. Thermodynamic data for the formation of complexes or compounds with hydroxide, chloride, fluoride, carbonate, nitrate, sulfate and phosphate are discussed in this report. The estimation of the stability constants by use of the Born equation is included. The Pitzer parameters for AnO and AnO
and AnO
 , redox potentials and equilibrium constants of redox reactions for actinides are also included.
, redox potentials and equilibrium constants of redox reactions for actinides are also included.
Yamaguchi, Tetsuji; Takeda, Seiji
JAERI-Data/Code 99-001, 74 Pages, 1999/01
no abstracts in English
 ZrO
ZrO packed bed
packed bedKawamura, Yoshinori; Enoeda, Mikio; Okuno, Kenji
Fusion Engineering and Design, 39-40, p.713 - 721, 1998/00
Times Cited Count:8 Percentile:56.56(Nuclear Science & Technology)no abstracts in English
Yamaguchi, Tetsuji;
Hoshasei Haikibutsu Kenkyu, 3(1), p.49 - 61, 1996/08
no abstracts in English