Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sano, Kyohei; Tameta, Yuito; Akuzawa, Tadashi; Kato, Soma; Takano, Yugo*; Akiyama, Kazuki
JAEA-Technology 2024-018, 68 Pages, 2025/02
High Active Solid Waste Storage Facility (HASWS) at the Tokai Reprocessing Plant (TRP) is a facility for storing highly radioactive solid waste generated from the reprocessing operation. Wet cells in HASWS store hull cans that contain fuel cladding tubes (hull) and fuel end pieces remained after the spent nuclear fuel shearing and dissolving, as well as used filters and contaminated equipment. Dry cells in HASWS store analytical waste containers that contain waste jugs and the other waste generated from analytical operation of samples in TRP. Since HASWS does not have waste recovery equipment from the cells, it is considered that recovery equipment to be installed. In the wet cells, methods of recovery wet-stored waste are being considered that utilize a ROV, which has been used in decommissioning in the UK, and a lifter, which is used in the marine industry to float and transport items sinking to the bottom of the sea. To confirm the feasibility of the recovery method that combines the functions of the ROV and the lifter, tests for removing waste were conducted in steps that came closer to the real environment: a "unit test" to confirm the functions required of each of the ROV and the lifter, a "combination test" to combine the ROV and the lifter to move waste underwater, and a "comprehensive test" to retrieve waste in an environment simulating the hull storage facility. Through this test, the ROV and the lifter were able to perform a series of tasks required to recovery waste - cutting the wires attached to the waste, attaching a lifter to the waste, moving the waste to under the opening, and attaching the recovery device to the moved waste - in series, confirming the feasibility of the method for recovery wet-stored waste using the ROV and the lifter.
Katsuoka, Nanako; Akiyama, Daisuke*; Kirishima, Akira*; Nagai, Takayuki; Okamoto, Yoshihiro; Sato, Nobuaki*
2023-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2024/07
no abstracts in English
 Cs in a river-sea system boundary area after the Fukushima Dai-ichi Nuclear Power Plant accident
Cs in a river-sea system boundary area after the Fukushima Dai-ichi Nuclear Power Plant accidentTakata, Hyoe*; Wakiyama, Yoshifumi*; Wada, Toshihiro*; Hirao, Shigekazu*; Aono, Tatsuo*; Nakanishi, Takahiro; Misono, Toshiharu; Shiribiki, Takehiko; Aoyama, Michio*
Marine Chemistry, 262, p.104384_1 - 104384_6, 2024/05
Times Cited Count:1 Percentile:45.28(Chemistry, Multidisciplinary)Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:2 Percentile:46.61(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
Nagai, Takayuki; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2023 (Internet), 3 Pages, 2024/00
no abstracts in English
Koyama, Shinichi; Ikeuchi, Hirotomo; Mitsugi, Takeshi; Maeda, Koji; Sasaki, Shinji; Onishi, Takashi; Tsai, T.-H.; Takano, Masahide; Fukaya, Hiroyuki; Nakamura, Satoshi; et al.
Hairo, Osensui, Shorisui Taisaku Jigyo Jimukyoku Homu Peji (Internet), 216 Pages, 2023/11
In FY 2021 and 2022, JAEA perfomed the subsidy program for "the Project of Decommissioning and Contaminated Water Management (Development of Analysis and Estimation Technology for Characterization of Fuel Debris (Development of Technologies for Enhanced Analysis Accuracy, Thermal Bahavior Estimation, and Simplified Analysis of Fuel Debris)" started in FY 2021. This presentation material summarized the results of the project, which will be available shortly on the website of Management Office for the Project of Decommissioning, Contaminated Water and Treated Water Management.
Akiyama, Yoichi; Shibanuma, So; Yanagisawa, Kenichi*; Yamada, Taichi; Suzuki, Kenta; Yoshida, Moeka; Ono, Takahiro; Kawabata, Kuniaki; Watanabe, Kaho; Morimoto, Kyoichi; et al.
JAEA-Review 2023-015, 60 Pages, 2023/09
Naraha Center for Remote Control Technology Development (NARREC) was established in Japan Atomic Energy Agency to promote a decommissioning work of Fukushima Daiichi Nuclear Power Station (Fukushima Daiichi NPS). NARREC consists of a Full-scale Mock-up Test Building and Research Management Building. Various test facilities are installed in these buildings for the decommissioning work of Fukushima Daiichi NPS. These test facilities are intended to be used for various users, such as companies engaged in the decommissioning work, research and development institutions, educational institutions and so on. The number of NARREC facility uses was 84 in FY2021. We participated booth exhibitions and presentations on the decommissioning related events. Moreover, we also contributed to the development of human resources by supporting the 6th Creative Robot Contest for Decommissioning. As a new project, "Narahakko Children's Classroom" was implemented for elementary school students in Naraha Town. This report summarizes the activities of NARREC in FY2021, such as the utilization of facilities and equipment of NARREC, the development of remote-control technologies for supporting the decommissioning work, arrangement of the remote-control machines for emergency response, and training for operators by using the machines.
Nagai, Takayuki; Akiyama, Daisuke*; Kirishima, Akira*; Katsuoka, Nanako; Okamoto, Yoshihiro; Sato, Nobuaki*
2022-Nendo "Busshitsu, Debaisu Ryoiki Kyodo Kenkyu Kyoten" Kenkyu Seika, Katsudo Hokokusho (Internet), 1 Pages, 2023/09
no abstracts in English
 dissolution in bicarbonate solution with H
dissolution in bicarbonate solution with H O
O ; The Effect of temperature
; The Effect of temperatureMcGrady, J.; Kumagai, Yuta; Kitatsuji, Yoshihiro; Kirishima, Akira*; Akiyama, Daisuke*; Watanabe, Masayuki
RSC Advances (Internet), 13(40), p.28021 - 28029, 2023/09
Times Cited Count:2 Percentile:22.93(Chemistry, Multidisciplinary)Upon nuclear waste canister failure and contact of spent nuclear fuel with groundwater, the UO matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
 ) is often found in groundwater, and the H
) is often found in groundwater, and the H O
O induced oxidative dissolution of UO
induced oxidative dissolution of UO in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H
in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H O
O at the UO
at the UO surface and dissolution of U
surface and dissolution of U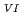 in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60
in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60  C). At [HCO
C). At [HCO
 ]
] 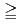 1 mM, the apparent equilibrium concentration of U
1 mM, the apparent equilibrium concentration of U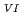 decreased with increasing temperature. This was attributed to the formation of U
decreased with increasing temperature. This was attributed to the formation of U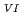 -bicarbonate species at the surface and a change in the mechanism of H
-bicarbonate species at the surface and a change in the mechanism of H O
O decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO
decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO in bicarbonate solution as a function of temperature was proposed.
in bicarbonate solution as a function of temperature was proposed.
Kusaka, Ryoji; Kumagai, Yuta; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 60(5), p.603 - 613, 2023/05
Times Cited Count:5 Percentile:61.74(Nuclear Science & Technology) O
O ) by mechanochemical treatment
) by mechanochemical treatmentAkiyama, Daisuke*; Mishima, Tomoki*; Okamoto, Yoshihiro; Kirishima, Akira*
High Temperature Materials and Processes, 42(1), p.20220268_1 - 20220268_9, 2023/04
A powder mixture of UO and TiO
and TiO was mechanochemically treated in a planetary ball mill under Ar atmosphere for 1 h using a tungsten carbide vial and balls as the milling medium. Such mechanochemical (MC) treatment reduced the crystallinity of UO
was mechanochemically treated in a planetary ball mill under Ar atmosphere for 1 h using a tungsten carbide vial and balls as the milling medium. Such mechanochemical (MC) treatment reduced the crystallinity of UO and TiO
and TiO . The mechanochemically treated powder mixture was heated at 973-1573K for 6 h under Ar atmosphere and analyzed by X-ray diffraction analysis, scanning electron microscopy-energy-dispersive X-ray spectroscopy, and X-ray absorption fine structure analysis. UTi
. The mechanochemically treated powder mixture was heated at 973-1573K for 6 h under Ar atmosphere and analyzed by X-ray diffraction analysis, scanning electron microscopy-energy-dispersive X-ray spectroscopy, and X-ray absorption fine structure analysis. UTi O
O did not form below 1373K without MC treatment and only the starting materials were observed. At 1473 and 1573K, a small amount of UTi
did not form below 1373K without MC treatment and only the starting materials were observed. At 1473 and 1573K, a small amount of UTi O
O and equal amounts of UTi
and equal amounts of UTi O
O and UO
and UO were formed, respectively. The mechanochemically treated sample produced nearly pure UTi
were formed, respectively. The mechanochemically treated sample produced nearly pure UTi O
O containing small amounts of UO
containing small amounts of UO impurities when heated above 1173K for 6 h. UTi
impurities when heated above 1173K for 6 h. UTi O
O was highly crystalline and uniform regardless of the synthesis temperature.
was highly crystalline and uniform regardless of the synthesis temperature.
 , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:6 Percentile:80.64(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O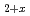 solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
Nagai, Takayuki; Tone, Masaya; Katsuoka, Nanako; Okamoto, Yoshihiro; Baba, Yuji*; Akiyama, Daisuke*
Photon Factory Activity Report 2022 (Internet), 3 Pages, 2023/00
no abstracts in English
 -edge to assess the U(V) electronic structure in FeUO
-edge to assess the U(V) electronic structure in FeUO
Yomogida, Takumi; Akiyama, Daisuke*; Ouchi, Kazuki; Kumagai, Yuta; Higashi, Kotaro*; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kawamura, Naomi*; Takahashi, Yoshio*
Inorganic Chemistry, 61(50), p.20206 - 20210, 2022/12
Times Cited Count:7 Percentile:59.79(Chemistry, Inorganic & Nuclear)FeUO was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L
was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L -edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
-edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:4 Percentile:53.26(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe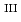 with increasing electron densities around Fe
with increasing electron densities around Fe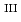 , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe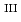
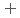 U
U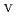
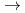 Fe
Fe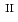
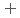 U
U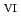 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr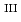 could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr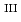 in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
Sato, Tomonori; Hata, Kuniki; Kaji, Yoshiyuki; Taguchi, Mitsumasa*; Seito, Hajime*; Inoue, Hiroyuki*; Tada, Eiji*; Abe, Hiroshi*; Akiyama, Eiji*; Suzuki, Shunichi*
Isotope News, (782), p.40 - 44, 2022/08
The stagnant water in the reactor building at Fukushima Daiichi Nuclear Power Station (1F) is exposed to the radiation from fuel debris and radioactive species. This water contains much amounts of impurities from the seawater which was injected in the emergency cooling. The impurities will affect the radiolysis and corrosive conditions in the water under irradiation. So, the water radiolysis data, corrosion data of steels under irradiations, and the evaluated potential impacts of corrosion in the decommissioning process of 1F are arranged as the database for corrosion under irradiation. This paper introduces the outline of this database.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:9 Percentile:79.01(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres. 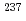 Np and
Np and 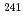 Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U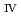 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:3 Percentile:42.88(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
Kirishima, Akira*; Terasaki, Mariko*; Miyakawa, Kazuya; Okamoto, Yoshihiro; Akiyama, Daisuke*
Chemosphere, 289, p.133181_1 - 133181_12, 2022/04
Times Cited Count:1 Percentile:3.54(Environmental Sciences)no abstracts in English
Furuike, Yoshihiko*; Ouyang, D.*; Tominaga, Taiki*; Matsuo, Tatsuhito*; Mukaiyama, Atsushi*; Kawakita, Yukinobu; Fujiwara, Satoru*; Akiyama, Shuji*
Communications Physics (Internet), 5(1), p.75_1 - 75_12, 2022/04
Times Cited Count:7 Percentile:59.84(Physics, Multidisciplinary)