Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
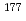 Lu for radioimmunotherapy
Lu for radioimmunotherapyWatanabe, Satoshi; Hashimoto, Kazuyuki; Watanabe, Shigeki; Iida, Yasuhiko*; Hanaoka, Hirofumi*; Endo, Keigo*; Ishioka, Noriko
Journal of Radioanalytical and Nuclear Chemistry, 303(1), p.935 - 940, 2015/01
Times Cited Count:16 Percentile:75.78(Chemistry, Analytical)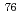 Br-labeled amino acid derivative for PET imaging of tumor
Br-labeled amino acid derivative for PET imaging of tumorHanaoka, Hirofumi*; Watanabe, Shigeki; Tominaga, Hideyuki*; Ohshima, Yasuhiro; Watanabe, Satoshi; Yamada, Keiichi*; Iida, Yasuhiko*; Ishioka, Noriko; Endo, Keigo*
JAEA-Review 2012-046, JAEA Takasaki Annual Report 2011, P. 89, 2013/01
no abstracts in English
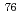 Br
BrWatanabe, Satoshi; Watanabe, Shigeki; Iida, Yasuhiko*; Hanaoka, Hirofumi*; Endo, Keigo*; Ishioka, Noriko
JAEA-Review 2011-043, JAEA Takasaki Annual Report 2010, P. 92, 2012/01
no abstracts in English
 Cu -labeled trastuzumab PET
Cu -labeled trastuzumab PETPaudyal, P.*; Paudyal, B.*; Hanaoka, Hirofumi*; Oriuchi, Noboru*; Iida, Yasuhiko*; Yoshioka, Hiroki*; Tominaga, Hideyuki*; Watanabe, Satoshi; Watanabe, Shigeki; Ishioka, Noriko; et al.
JAEA-Review 2010-065, JAEA Takasaki Annual Report 2009, P. 108, 2011/01
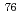 Br-
Br-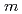 -bromobenzylguanidine (
-bromobenzylguanidine (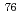 Br-MBBG)
Br-MBBG)Watanabe, Shigeki; Hanaoka, Hirofumi*; Liang, J. X.*; Iida, Yasuhiko*; Watanabe, Satoshi; Endo, Keigo*; Ishioka, Noriko
JAEA-Review 2010-065, JAEA Takasaki Annual Report 2009, P. 107, 2011/01
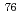 Br-
Br- -bromobenzylguanidine
-bromobenzylguanidineWatanabe, Shigeki; Hanaoka, Hirofumi*; Liang, J. X.; Iida, Yasuhiko*; Endo, Keigo*; Ishioka, Noriko
Journal of Nuclear Medicine, 51(9), p.1472 - 1479, 2010/09
Times Cited Count:22 Percentile:56.72(Radiology, Nuclear Medicine & Medical Imaging) Cu-labeled trastuzumab PET
Cu-labeled trastuzumab PETPaudyal, P.*; Paudyal, B.*; Hanaoka, Hirofumi*; Oriuchi, Noboru*; Iida, Yasuhiko*; Yoshioka, Hiroki*; Tominaga, Hideyuki*; Watanabe, Satoshi; Watanabe, Shigeki; Ishioka, Noriko; et al.
Cancer Science, 101(4), p.1045 - 1050, 2010/04
Times Cited Count:41 Percentile:66.73(Oncology)Iida, Yasuhiko*; Hanaoka, Hirofumi*; Watanabe, Satoshi; Watanabe, Shigeki; Ishioka, Noriko; Yoshioka, Hiroki*; Yamamoto, Shinji*; Paudyal, P.*; Paudyal, B.*; Higuchi, Tetsuya*; et al.
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 108, 2009/12
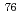 Br-m-bromobenzylguanidine (
Br-m-bromobenzylguanidine (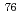 Br-MBBG) for in vivo imaging of neuroendcrine-tumor with PET
Br-MBBG) for in vivo imaging of neuroendcrine-tumor with PETWatanabe, Shigeki; Hanaoka, Hirofumi*; Liang, J. X.; Iida, Yasuhiko*; Watanabe, Satoshi; Endo, Keigo*; Ishioka, Noriko
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 107, 2009/12
Watanabe, Satoshi; Hashimoto, Kazuyuki; Watanabe, Shigeki; Iida, Yasuhiko*; Hanaoka, Hirofumi*; Endo, Keigo*; Ishioka, Noriko
JAEA-Review 2009-041, JAEA Takasaki Annual Report 2008, P. 109, 2009/12
no abstracts in English
 Cu-labeled DOTA-
Cu-labeled DOTA-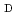 -Phe
-Phe -Tyr
-Tyr -octreotide (
-octreotide ( Cu-DOTA-TOC) for imaging somatostatin receptor-expressing tumors
Cu-DOTA-TOC) for imaging somatostatin receptor-expressing tumorsHanaoka, Hirofumi*; Tominaga, Hideyuki*; Yamada, Keiichi*; Paudyal, P.*; Iida, Yasuhiko*; Watanabe, Shigeki; Paudyal, B.*; Higuchi, Tetsuya*; Oriuchi, Noboru*; Endo, Keigo*
Annals of Nuclear Medicine, 23(6), p.559 - 567, 2009/08
Times Cited Count:19 Percentile:50.56(Radiology, Nuclear Medicine & Medical Imaging) Cu and applications to molecular imaging by PET and PETIS as a biomedical tracer
Cu and applications to molecular imaging by PET and PETIS as a biomedical tracerWatanabe, Shigeki; Iida, Yasuhiko*; Suzui, Nobuo; Katabuchi, Tatsuya*; Ishii, Satomi; Kawachi, Naoki; Hanaoka, Hirofumi*; Watanabe, Satoshi; Matsuhashi, Shimpei; Endo, Keigo*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 280(1), p.199 - 205, 2009/04
Times Cited Count:25 Percentile:81.97(Chemistry, Analytical)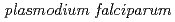 enolase
enolaseOku, Hiroyuki*; Yamada, Keiichi*; Kobayashi, Kyoko*; Katakai, Ryoichi*; Ashfaq, M.*; Hanaoka, Hirofumi*; Iida, Yasuhiko*; Endo, Keigo*; Hasegawa, Shin; Maekawa, Yasunari; et al.
Peptide Science 2008, p.439 - 442, 2009/03
Malaria is the major cause of mortality and morbidity in the tropical and subtropical regions in the world. Our previous studies have shown that a series of partial peptides of a Plasmodium falciparum enolase have antigenic reactivity against patients' sera. In this paper, we wish to report nano-encapsulation of a synthetic antigen into bioabsorbable polymer particles and their releasing studies in vitro and in vivo.
Iida, Yasuhiko*; Hanaoka, Hirofumi*; Paudyal, P.*; Paudyal, B.*; Watanabe, Satoshi; Ishioka, Noriko; Watanabe, Shigeki; Matsuhashi, Shimpei; Yoshioka, Hiroki*; Higuchi, Tetsuya*; et al.
JAEA-Review 2008-055, JAEA Takasaki Annual Report 2007, P. 114, 2008/11
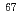 Cu via the
Cu via the 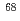 Zn(p,2p)
Zn(p,2p) 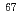 Cu reaction and recovery of
Cu reaction and recovery of 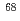 Zn target
Zn targetKatabuchi, Tatsuya*; Watanabe, Satoshi; Ishioka, Noriko; Iida, Yasuhiko*; Hanaoka, Hirofumi*; Endo, Keigo*; Matsuhashi, Shimpei
Journal of Radioanalytical and Nuclear Chemistry, 277(2), p.467 - 470, 2008/08
Times Cited Count:36 Percentile:88.65(Chemistry, Analytical)Iida, Yasuhiko*; Hanaoka, Hirofumi*; Katabuchi, Tatsuya*; Watanabe, Shigeki; Ishioka, Noriko; Watanabe, Satoshi; Matsuhashi, Shimpei; Higuchi, Tetsuya*; Oriuchi, Noboru*; Endo, Keigo*
JAEA-Review 2007-060, JAEA Takasaki Annual Report 2006, P. 128, 2008/03
Iida, Yasuhiko*; Hanaoka, Hirofumi*; Katabuchi, Tatsuya*; Watanabe, Satoshi; Ishioka, Noriko; Matsuhashi, Shimpei; Oriuchi, Noboru*; Higuchi, Tetsuya*; Miyakubo, Mitsuyuki*; Endo, Keigo*
JAEA-Review 2006-042, JAEA Takasaki Annual Report 2005, P. 165, 2007/02
Katabuchi, Tatsuya*; Watanabe, Satoshi; Ishioka, Noriko; Matsuhashi, Shimpei; Iida, Yasuhiko*; Hanaoka, Hirofumi*; Endo, Keigo*
JAEA-Review 2006-042, JAEA Takasaki Annual Report 2005, P. 132, 2007/02
no abstracts in English
 Re]Organorhenium-labeled antibody fragments with renal enzyme-cleavable linkage for low renal radioactivity levels
Re]Organorhenium-labeled antibody fragments with renal enzyme-cleavable linkage for low renal radioactivity levelsUehara, Tomoya*; Koike, Miho*; Nakata, Hideo*; Hanaoka, Hirofumi*; Iida, Yasuhiko*; Hashimoto, Kazuyuki; Akizawa, Hiromichi*; Endo, Keigo*; Arano, Yasushi*
Bioconjugate Chemistry, 18(1), p.190 - 198, 2007/01
Times Cited Count:23 Percentile:57.65(Biochemical Research Methods)Renal localization of radiolabeled antibody fragments constitutes a problem in targeted imaging and radiotherapy. To estimate the applicability of the molecular design to metallic radionuclides, [ Re]tricarbonyl(cyclopentadienylcarbonate)rhenium ([
Re]tricarbonyl(cyclopentadienylcarbonate)rhenium ([ Re]CpTR-COOH) was conjugated with maleoyl-glycyl-lysine to prepare [
Re]CpTR-COOH) was conjugated with maleoyl-glycyl-lysine to prepare [ Re]CpTR-GK. The cleavage of the glycyl-lysine linkage of the compound generates a glycine conjugate of [
Re]CpTR-GK. The cleavage of the glycyl-lysine linkage of the compound generates a glycine conjugate of [ Re]CpTR-Gly. [
Re]CpTR-Gly. [ Re]CpTR-GK was conjugated to thiolated Fab fragments to prepare [
Re]CpTR-GK was conjugated to thiolated Fab fragments to prepare [ Re]CpTR-GK-Fab. The biodistribution of radioactivity after injection of [
Re]CpTR-GK-Fab. The biodistribution of radioactivity after injection of [ Re]CpTR-GK-Fab was compared with that of [
Re]CpTR-GK-Fab was compared with that of [ Re]CpTR-Fab. [
Re]CpTR-Fab. [ Re]CpTR-GK-Fab exhibited significantly lower renal radioactivity levels than did [
Re]CpTR-GK-Fab exhibited significantly lower renal radioactivity levels than did [ Re]CpTR-Fab. The analysis of urine samples collected for 6 h postinjection of [
Re]CpTR-Fab. The analysis of urine samples collected for 6 h postinjection of [ Re]CpTR-GK-Fab showed that [
Re]CpTR-GK-Fab showed that [ Re]CpTR-Gly was the major radiometabolite. In tumor-bearing mice, [
Re]CpTR-Gly was the major radiometabolite. In tumor-bearing mice, [ Re]CpTR-GK-Fab significantly reduced renal radioactivity levels without impairing the radioactivity levels in tumor. These findings indicate that the molecular design of radioparmaceuticals labeled with metallic radionuclides can be useful by using a radiometal chelate of high inertness and by designing a radiometabolite of high urinary excretion when released from antibody fragments following cleavage of a glycyl-lysine linkage. This study also indicates that a change in chemical structure of a radiolabel attached to a glycyl-lysine linkage significantly affected enzymes involved in the hydrolysis reaction.
Re]CpTR-GK-Fab significantly reduced renal radioactivity levels without impairing the radioactivity levels in tumor. These findings indicate that the molecular design of radioparmaceuticals labeled with metallic radionuclides can be useful by using a radiometal chelate of high inertness and by designing a radiometabolite of high urinary excretion when released from antibody fragments following cleavage of a glycyl-lysine linkage. This study also indicates that a change in chemical structure of a radiolabel attached to a glycyl-lysine linkage significantly affected enzymes involved in the hydrolysis reaction.
Kambara, Toyozo; Uno, Hidero; Shoda, Katsuhiko; Hirata, Yutaka; Shoji, Tsutomu; Kohayakawa, Toru; Takayanagi, Hiroshi; Fujimura, Tsutomu; Morita, Morito; Ichihara, Masahiro; et al.
JAERI 1045, 11 Pages, 1963/03
no abstracts in English