Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakama, Shigeo; Yoshimura, Kazuya; Fujiwara, Kenso; Ishikawa, Hiroyasu; Iijima, Kazuki
Journal of Environmental Radioactivity, 208-209, p.106013_1 - 106013_8, 2019/11
Times Cited Count:9 Percentile:29.88(Environmental Sciences)Trends of air dose rate decrease after decontamination works and factors which affect them constitute essential information for radiation protection, such as prediction of external exposure to the public and implementation of measures to reduce such exposure. This study investigated the decrease of air dose rate (ambient dose rate at 1 m above the ground) at 163 points across sub-urban areas in the evacuation zone around the Fukushima Dai-ichi Nuclear Power Plant over the period of four years following the decontamination works carried out in November 2012. The air dose rate on the asphalt pavement decreased faster than on soil surfaces. In addition, air dose rates near the forest decreased at a slower pace than in open fields. These results suggest that the air dose rate in urbanized areas can decrease faster than in other types of land, even after decontamination. Based on comparisons with decrease rates obtained in other studies, the air dose rate tends to decrease faster outside the evacuation zone than inside it. The decrease in air dose rate after decontamination was slower than before decontamination. The contribution of the weathering effect and human activity was estimated to be about 80% and 20% of the ecological decrease rate, respectively.
Nakama, Shigeo; Yoshimura, Kazuya; Fujiwara, Kenso; Ishikawa, Hiroyasu; Iijima, Kazuki
KEK Proceedings 2018-7, p.154 - 158, 2018/11
Decrease in air dose rate in decontaminated area is essential information to estimate external exposure and to facilitate return of local residents, but the factors to control the decrease rate have not been cleared wholly. To clarify the effect of ground surface type (i.e. paved and soil surfaces) on the decrease in air dose rate at 1 m above the ground, surface dose rate at 1 cm above the ground and the air dose rate were monitored for four years since decontamination in 2011, and their decrease rates were compared relating to the ground surface type. Decrease in the air dose rate and the surface dose rate on the asphalt pavement showed faster rates than those on the soil surface. Ratio of decrease in the air dose rate and surface dose rate (decrease rate ratio) was distributed between 0.8 and 1.2 on open place not affected by surrounding environment. Therefore, decrease in the air dose rate was in agreement with the decrease of the surface dose rate, which is greatly affected by the ground surface. It became clear that the decrease rate constant of the air dose rate differs depending on the difference in the ground surface. Furthermore, it was also confirmed that the local soil erosion and sedimentation of the ground surface does not affect the decrease rate of the air dose rate.
 -ray camera
-ray cameraTakeda, Shinichiro*; Ichinohe, Yuto*; Hagino, Koichi*; Odaka, Hirokazu*; Yuasa, Takayuki*; Ishikawa, Shinnosuke*; Fukuyama, Taro*; Saito, Shinya*; Sato, Tamotsu*; Sato, Goro*; et al.
Physics Procedia, 37, p.859 - 866, 2012/10
Times Cited Count:25 Percentile:98.53(Physics, Applied)By using new Compton camera consisting of silicon double-sided strip detector (Si-DSD) and CdTe-DSD developed for the ASTRO-H mission, an experiment was conducted to study its feasibility for advanced hotspot monitoring. In addition to hotspot imaging already provided by commercial imaging systems, the identification of the variety of radioisotopes is realized thanks to the good energy resolution given by the semiconductor detectors. Three radioisotopes of  Ba (356 keV),
Ba (356 keV),  Na (511 keV) and
Na (511 keV) and  Cs (662 keV) were individually imaged by applying event selection in the energy window and the
Cs (662 keV) were individually imaged by applying event selection in the energy window and the  -ray images was correctly overlapped by an optical picture. The detection efficiency of 1.68
-ray images was correctly overlapped by an optical picture. The detection efficiency of 1.68 10
10 (effective area: 1.7
(effective area: 1.7 10
10 cm
cm ) and angular resolution of 3.8
) and angular resolution of 3.8 were obtained by stacking five detector modules for 662 keV
were obtained by stacking five detector modules for 662 keV  -ray. The higher detection efficiency required in a specific use can be achieved by stacking more detector modules.
-ray. The higher detection efficiency required in a specific use can be achieved by stacking more detector modules.
Kisohara, Naoyuki; Ishikawa, Hiroyasu; Futagami, Satoshi; Xu, Y.*; Shimoji, Kuniyuki*; Kawamura, Masaya*
Proceedings of 19th International Conference on Nuclear Engineering (ICONE-19) (CD-ROM), 9 Pages, 2011/10
The cooling system of the JSFR adopts an integrated primary sodium pump/intermediate heat exchanger (IHX), dual structure straight tube steam generator (SG) and short elbow sodium piping layout. Since, however, this is the first experience applying these technologies to SFRs in Japan, design approaches have been evaluated and R&D has been undertaken. This paper addresses the design study of the cooling system of the demonstration reactor JSFR in terms of thermal-hydraulic and structural integrity. Recent studies have shown that these new technologies have potential to be applied to the JSFR.
Miyahara, Shinya; Ishikawa, Hiroyasu; Yoshizawa, Yoshio*
Nuclear Engineering and Design, 241(5), p.1319 - 1328, 2011/05
Times Cited Count:15 Percentile:71.97(Nuclear Science & Technology)Reaction behavior of carbon dioxide (CO ) with a liquid sodium pool was experimentally investigated to understand the consequences of boundary tube failure in a sodium-CO
) with a liquid sodium pool was experimentally investigated to understand the consequences of boundary tube failure in a sodium-CO heat exchanger (HX). In this study, two kinds of experiments, namely fundamental experiment and demonstration experiment which simulate the incident of CO
heat exchanger (HX). In this study, two kinds of experiments, namely fundamental experiment and demonstration experiment which simulate the incident of CO leakage in HX, were carried out to investigate the reaction behavior. From these experiments, it became clear that the exothermic reaction occurred above a threshold temperature, and useful and indispensable information such as the resulting temperature and pressure rise and the behavior of solid reaction products in the pool was obtained to evaluate the consequences of boundary tube failure incident in the sodium- CO
leakage in HX, were carried out to investigate the reaction behavior. From these experiments, it became clear that the exothermic reaction occurred above a threshold temperature, and useful and indispensable information such as the resulting temperature and pressure rise and the behavior of solid reaction products in the pool was obtained to evaluate the consequences of boundary tube failure incident in the sodium- CO heat exchanger.
heat exchanger.
 -ray imaging capability with a Si/CdTe semiconductor Compton camera
-ray imaging capability with a Si/CdTe semiconductor Compton cameraTakeda, Shinichiro*; Aono, Hiroyuki*; Okuyama, Sho*; Ishikawa, Shinnosuke*; Odaka, Hirokazu*; Watanabe, Shin*; Kokubun, Motohide*; Takahashi, Tadayuki*; Nakazawa, Kazuhiro*; Tajima, Hiroyasu*; et al.
IEEE Transactions on Nuclear Science, 56(3), p.783 - 790, 2009/06
Times Cited Count:57 Percentile:95.72(Engineering, Electrical & Electronic)Miyahara, Shinya; Ishikawa, Hiroyasu; Yoshizawa, Yoshio*
Proceedings of 17th International Conference on Nuclear Engineering (ICONE-17) (CD-ROM), 8 Pages, 2009/06
Reaction behavior of carbon dioxide (CO ) with a liquid sodium pool was experimentally investigated to understand the consequences of boundary tube failure in a sodium-CO
) with a liquid sodium pool was experimentally investigated to understand the consequences of boundary tube failure in a sodium-CO heat exchanger. In this study, two kinds of experiments were carried out to investigate the reaction behavior. In one experiment, about 1-5 g of liquid sodium pool were poured into flowing CO
heat exchanger. In this study, two kinds of experiments were carried out to investigate the reaction behavior. In one experiment, about 1-5 g of liquid sodium pool were poured into flowing CO to obtain the information mainly about the thermo-chemical conditions to initiate the reaction and the chemical constituents of reaction products. During the experiment, visual observation was made using video-camera and the temperature change of the sodium pool and near the surface was measured by thermocouples. In the other experiment, CO
to obtain the information mainly about the thermo-chemical conditions to initiate the reaction and the chemical constituents of reaction products. During the experiment, visual observation was made using video-camera and the temperature change of the sodium pool and near the surface was measured by thermocouples. In the other experiment, CO was injected into about 200 g of liquid sodium pool to simulate the boundary failure in the sodium-CO
was injected into about 200 g of liquid sodium pool to simulate the boundary failure in the sodium-CO heat exchanger. The temperature change of sodium pool and the cover gas was measured by thermocouples during the experiment. From these experiments, it became clear that the exothermic reaction occurred above a threshold temperature, and useful and indispensable information such as the resulting temperature and pressure rise and the behavior of solid reaction products in the pool was obtained to evaluate the consequences of boundary tube failure incident in a sodium-CO
heat exchanger. The temperature change of sodium pool and the cover gas was measured by thermocouples during the experiment. From these experiments, it became clear that the exothermic reaction occurred above a threshold temperature, and useful and indispensable information such as the resulting temperature and pressure rise and the behavior of solid reaction products in the pool was obtained to evaluate the consequences of boundary tube failure incident in a sodium-CO heat exchanger.
heat exchanger.
Ishikawa, Hiroyasu; Miyahara, Shinya; Yoshizawa, Yoshio*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 7(4), p.452 - 461, 2008/12
Supercritical CO is being investigated as a material for a secondary cooling system of Na-cooled fast reactor in to avoid Na/water reaction. In this type of reactor, however, it is necessary to consider the consequences of Na/CO
is being investigated as a material for a secondary cooling system of Na-cooled fast reactor in to avoid Na/water reaction. In this type of reactor, however, it is necessary to consider the consequences of Na/CO reaction, which might occur in the case of tube rupture in a heat exchanger between primary and secondary systems. Experiments were carried out with test equipment for the Na/CO
reaction, which might occur in the case of tube rupture in a heat exchanger between primary and secondary systems. Experiments were carried out with test equipment for the Na/CO reaction, which can handle 1-5 g order of Na. The solid products of the Na/CO
reaction, which can handle 1-5 g order of Na. The solid products of the Na/CO reaction sampled from the equipment were analyzed by XRD and chemical analysis. From these experimental results, we proved that the reaction proceeded between liquid Na and CO
reaction sampled from the equipment were analyzed by XRD and chemical analysis. From these experimental results, we proved that the reaction proceeded between liquid Na and CO . The Na/CO
. The Na/CO reaction stopped only the pool surface reaction with a small quantity of aerosol emission when the temperature was lower than 570
reaction stopped only the pool surface reaction with a small quantity of aerosol emission when the temperature was lower than 570 C. On the other hand, the reaction continuously proceeded with on orange-colored flame and aerosol release when the temperature was higher than 580
C. On the other hand, the reaction continuously proceeded with on orange-colored flame and aerosol release when the temperature was higher than 580 C.
C.
Katayama, Kazunari*; Takeishi, Toshiharu*; Nagase, Hiroyasu*; Manabe, Yusuke*; Nishikawa, Masabumi*; Miya, Naoyuki; Masaki, Kei
Fusion Science and Technology, 48(1), p.561 - 564, 2005/07
Times Cited Count:1 Percentile:10.20(Nuclear Science & Technology)no abstracts in English
Ishikawa, Hiroyasu; Miyahara, Shinya; Yoshizawa, Yoshio*
Proceedings of 2005 International Congress on Advances in Nuclear Power Plants (ICAPP '05) (CD-ROM), P. 5688, 2005/05
Focusing on the cover layer materials (as the Radon Barrier Materials), which could have the effect to restrain the radon from scattering into the air and the effect of the radiation shielding, we produced the radon barrier materials with crude bentonite on an experimental basis, using the rotary type comprehensive unit for grinding and mixing, through which we carried out the evaluation of the characteristics thereof.
Doda, Norihiro; Ishikawa, Hiroyasu; Ohno, Shuji; Miyahara, Shinya
JNC TN9400 2005-048, 52 Pages, 2005/04
The burning behavior of a single sodium droplet has been studied for understanding of spray combustion which is one of the combustion forms in sodium leakage. This study serves as the basis of the mechanistic sodium fire analysis method. A burning experiment with initial droplet size of 3.34mm and 5.85mm different from FD-2 experiment condition (4.75mm), an inert gas condition experiment and a polypropylene sphere experiment were performed to investigate the relation between initial droplet size and burnt mass, and the effect of burning phenomena on the droplet motion. (1)Drag coefficient of a burning sodiuum droplet is 1.4 2.2 times greater than that of a solid sphere of the same size. (2)The increase in drag force of a burning sodium droplet is mainly due to the increase in gaseous viscosity around the droplet with heat of combustion. Sodium droplet has the ellipsoidal shape with aspect ratio 0.89 when falling by about 8 meters, but the effect of droplet deformation is negligible small. In addition, evaporation and buoyancy have also little effect on the increase in drag force. (3)The burning of sodium droplet follows the D
2.2 times greater than that of a solid sphere of the same size. (2)The increase in drag force of a burning sodium droplet is mainly due to the increase in gaseous viscosity around the droplet with heat of combustion. Sodium droplet has the ellipsoidal shape with aspect ratio 0.89 when falling by about 8 meters, but the effect of droplet deformation is negligible small. In addition, evaporation and buoyancy have also little effect on the increase in drag force. (3)The burning of sodium droplet follows the D -law when the initial droplet diameter changes. In the calculation which assumes that the sodium combustion quantity agrees with the measurement and that combustion of sodium droplet obeys that law, the Na
-law when the initial droplet diameter changes. In the calculation which assumes that the sodium combustion quantity agrees with the measurement and that combustion of sodium droplet obeys that law, the Na O ratio of reaction products becomes 0.51-0.75.
O ratio of reaction products becomes 0.51-0.75.
Katayama, Kazunari*; Takeishi, Toshiharu*; Manabe, Yusuke*; Nagase, Hiroyasu*; Nishikawa, Masabumi*; Miya, Naoyuki
Journal of Nuclear Materials, 340(1), p.83 - 92, 2005/04
Times Cited Count:8 Percentile:48.00(Materials Science, Multidisciplinary)no abstracts in English
Ishikawa, Hiroyasu; Doda, Norihiro; Miyahara, Shinya
Kasai, 55(1), p.50 - 60, 2005/00
This paper is a report of sodium fire study in Japan Nuclear Cycle Development Institute.
Ishikawa, Hiroyasu; Ohno, Shuji; Miyahara, Shinya
JNC TN9400 2004-038, 84 Pages, 2004/04
Nitrogen gas can be an extinguisher or a mitigating material in the case of sodium leak and fire accident in an air atmosphere, which may occur at a liquid metal cooled nuclear power plant. However, sodium combustion residuum sometimes reignites in the air atmosphere even at room temperature when it was produced by nitrogen gas injection to the burning sodium. Then, in this study we executed conclusive experiments of prevention measures against sodium combustion residuum reignition by a mixture of carbon-dioxide (C0 sub2) gas, humidity and nitrogen gas. The experiments were carried out with the FRAT-1 test equipment; the humidity conditions were changed in air which were used to sodium combustion atmosphere and exposure air for confirmation of prevented combustion residue reignition. First of all, the sodium of about 2.5kg was leaked in air atmosphere, and next, the sodium combustion was stopped by nitrogen gas injection. Next, the combustion residuum was cooled in the nitrogen atmosphere, and then the combustion residuum was exposed to atmosphere of carbon-dioxide (4%); humidity (6000vppm); oxygen (3%)-nitrogen (based gas) mixture. It was confirmed that the combustion residuum was not reignition even if exposed to the air atmosphere again at the end of experiment. We had confirmed that the prevention measures against sodium combustion residuum reignition to establish by this research were effective.
 (
(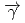 ,
,
 )
)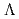 and
and  (
(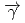 ,
,
 )
)
 reactions for
reactions for 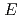
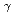 =1.5-2.4 GeV
=1.5-2.4 GeVZegers, R. G. T.*; Sumihama, Mizuki*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Dat , S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; et al.
Physical Review Letters, 91(9), p.092001_1 - 092001_4, 2003/08
Times Cited Count:128 Percentile:94.59(Physics, Multidisciplinary)no abstracts in English
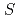 = +1 Baryon resonance in photoproduction from the neutron
= +1 Baryon resonance in photoproduction from the neutronNakano, Takashi*; Ahn, D. S.*; Ahn, J. K.*; Akimune, Hidetoshi*; Asano, Yoshihiro; Chang, W. C.*; Date, S.*; Ejiri, Hiroyasu*; Fujimura, Hisako*; Fujiwara, Mamoru; et al.
Physical Review Letters, 91(1), p.012002_1 - 012002_4, 2003/07
Times Cited Count:1023 Percentile:99.84(Physics, Multidisciplinary)no abstracts in English
Ishikawa, Hiroyasu; Ohno, Shuji; Miyahara, Shinya
JNC TN9400 2002-081, 46 Pages, 2003/01
Nitrogen gas can be an extinguisher or a mitigating material in the case of sodium leak and fire accident in an air atmosphere, which may occur at a liquid metal cooled nuclear power plant. However sodium combustion residuum sometimes reignites in the air atmosphere even at room temperature when it was produced by nitrogen gas injection to the burning sodium. In this study we have been investigating the cause of reignition and prevention measures. Experiments were carried out with small type test equipment, which can handle 1g order sodium fire and extinguishment. Sodium combustion residua, which were made by our equipment and sampled, were analyzed by X-ray diffraction and chemical analysis. The chemical analysis of reignitable residua showed that the residuum contained metallic sodium of about 40wt-% (61 mol-%) to 60-wt % (76mol-%) and most of the rest was sodium-monoxide (Na O). Sodium-peroxide (Na
O). Sodium-peroxide (Na O
O ) was also included in less than 1wt-% of the residuum. Sodium or Na
) was also included in less than 1wt-% of the residuum. Sodium or Na O cannot ignite by itself in the air atmosphere at room temperature in a few minutes. Therefore the reignition seems to be due to increase in the local temperature that is caused by oxidizing heat of Na and by adiabatic effect of Na
O cannot ignite by itself in the air atmosphere at room temperature in a few minutes. Therefore the reignition seems to be due to increase in the local temperature that is caused by oxidizing heat of Na and by adiabatic effect of Na O. It is important to deactivate this dispersed sodium on oxygen for prevention of the residuum reignition, hence it is considered as a rational measure to change the sodium to sodium-carbonate. Our experiments showed that the dispersed sodium on the exterior of residuum could be changed to carbonate by a mixture of carbon-dioxide (CO
O. It is important to deactivate this dispersed sodium on oxygen for prevention of the residuum reignition, hence it is considered as a rational measure to change the sodium to sodium-carbonate. Our experiments showed that the dispersed sodium on the exterior of residuum could be changed to carbonate by a mixture of carbon-dioxide (CO ) gas (2 to 8vol-%), humidity (0.6 to 3vol-%) and nitrogen gas. The deactivated residuum did not reignite in the air atmosphere below 473K.
) gas (2 to 8vol-%), humidity (0.6 to 3vol-%) and nitrogen gas. The deactivated residuum did not reignite in the air atmosphere below 473K.
Ishikawa, Hiroyasu; Ohno, Shuji; Miyahara, Shinya
Nihon Nensho Gakkai-Shi, 45(134), p.248 - 256, 2003/00
Nitrogen gas can be an extinguisher or a mitigating in the case of sodium leak and fire accident in an air atmonphere, which may occur at a liquid metal cooled nuclear power plant. However sodium combustion residuum sometimes reignites in the air atmosphere even at room temperature when it was produced by nitrogen gas injection to the burning sodium. In this study we have been investigating the cause of reignition and prevention measures. Experiments were carried out with small type test equipment, which can handle 1g order sodium fire and extinguishment. Sodium combustion residua, which were made by our equipment and sampled, were analyzed by X-ray diffraction and chemical analyses. The chemical analysis of reignitable residua showed that the residuum contained metallic sodium of about 40Wt-%(61mol-%)to 60-wt %(76mol-%) and most of the rest was sodium-monoxide (Na2O).Sodium-peroxide (Na2O2) was also included in less than 1wt-% of the residuu. Sodium or Na2O cannot ignite by itself in the air atmosphere at room temperature in a few minutes. Therefore the reignition seems to be due to increase in the local temperature that is caused by oxidizing heat of Na and by adiabatic effect of Na2O. It is important to deactivate this dispersed sodium on oxygen for prevention of the residuum reigntion, hence it is considered as a rational measure to change the sodium to sodium-carbonate. Our experments showed that the dispersed sodium on the exterior of residuum could be changed to carbonate by a mixture by a mixture of carbon-dioxide(CO2) gas (2 to 8vol-%),humidity (0.6 to 3vol-%) and mitrogen gas. The deactivated residuum did not reignite in the air atmospherebe bwlow 473K.
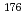 Yb
Yb
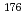 Lu for spectroscopy of proton-proton and other sub-MeV solar neutrinos
Lu for spectroscopy of proton-proton and other sub-MeV solar neutrinosFujiwara, Mamoru; Akimune, Hidetoshi*; Van den Berg, A. M.*; Cribier, M.*; Daito, Izuru*; Ejiri, Hiroyasu*; Fujimura, Hisako*; Fujita, Yoshitaka*; Goodman, C. D.*; Hara, Keigo*; et al.
Physical Review Letters, 85(21), p.4442 - 4445, 2000/11
Times Cited Count:24 Percentile:73.57(Physics, Multidisciplinary)no abstracts in English
Futagami, Satoshi; Nishimura, Masahiro; Kawata, Koji; Ishikawa, Hiroyasu; Miyahara, Shinya
PNC TN9410 98-074, 154 Pages, 1998/08
Supposing sodium leak accident from a IHTS pipe in FBR, sodium pool combusion test Run-F7 series is under way to know liner peak temperature and to study sodium pool growth and combustion behavior in the case of small-scale sodium leaks (0.01ton/hr) in an air atmosphere. This report consists of a result of Run-F7-1 (Feb,17,1998) and Run-F7-2 (Apr, 21, 1998) : In these tests, sodium temperature and sodium leak rates are the same (about 0.01ton/hr), but the height of leak point was changed. The test was performed using FRAT-1 (Fission Product and Radioactive Aerosol Release Test Rig . volume : 3m ) of SAPFIRE (Safety Phenomenology Tests on Sodium Leak, Fire and Aerosols) facility. A burning pan (diameter : about 1000 mm, thickness : 6 mm) made of carbon steel (SM400B) to simulate floor steal liner was installed in FRAT-1. a 5kg of sodium was leaked at the leak rate of 0.01ton/hr under the temperature of 507
) of SAPFIRE (Safety Phenomenology Tests on Sodium Leak, Fire and Aerosols) facility. A burning pan (diameter : about 1000 mm, thickness : 6 mm) made of carbon steel (SM400B) to simulate floor steal liner was installed in FRAT-1. a 5kg of sodium was leaked at the leak rate of 0.01ton/hr under the temperature of 507 C from the height of 0.1m and 1.5m (Run-F7-1 and Run-F7-2, respectively) during 30minutes when air was continued to feed into the cell. From the results, the following conclusion were drawn concerning the liner peak temperature, and sodium pool growth and combustion behavior, in the small-scale (0.01ton/hr) sodium leak: (1)In this case (1eak rate 0.01ton/hr), burning pan thermocouple measured 616
C from the height of 0.1m and 1.5m (Run-F7-1 and Run-F7-2, respectively) during 30minutes when air was continued to feed into the cell. From the results, the following conclusion were drawn concerning the liner peak temperature, and sodium pool growth and combustion behavior, in the small-scale (0.01ton/hr) sodium leak: (1)In this case (1eak rate 0.01ton/hr), burning pan thermocouple measured 616 C at approximately 33minutes in Run-F7-1, and 675
C at approximately 33minutes in Run-F7-1, and 675 C at 33minutes in Run-F7-2. therefore, the height of leak point has an effect on liner peak temperature. (2)Post test observation clarified that the deposits covered an area of about 0.3m
C at 33minutes in Run-F7-2. therefore, the height of leak point has an effect on liner peak temperature. (2)Post test observation clarified that the deposits covered an area of about 0.3m in both tests. Sodium pool growth velocity during experiments were also similar. therefore, the height of leak point doesn't have a notable effect on sodium pool growth. (3)In both tests, burning rate of a unit area was 16kg-Na/m
in both tests. Sodium pool growth velocity during experiments were also similar. therefore, the height of leak point doesn't have a notable effect on sodium pool growth. (3)In both tests, burning rate of a unit area was 16kg-Na/m hr. (4)Analysis of the chemical composition of the deposits on the burning pan revealed that the main compounds were sodium oxides, but sodium dioxide and ...
hr. (4)Analysis of the chemical composition of the deposits on the burning pan revealed that the main compounds were sodium oxides, but sodium dioxide and ...