Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Asahi, Yoshimitsu; Fukuda, Shigeki; Shiramizu, Daiki; Miyata, Koshi; Tone, Masaya; Katsuoka, Nanako; Maeda, Yuta; Aoyama, Yusuke; Niitsuma, Koichi; Kobayashi, Hidekazu; et al.
JAEA-Technology 2024-024, 271 Pages, 2025/03
A glass melter for the vitrification process of highly active liquid waste in the Tokai Reprocessing Plant, TVF's 3rd melter, was built, and the glass of 18 vitrified waste canisters in weight was melted and poured through a cold test operation. The molten glass surface was covered by a cold cap from feeding fiberglass cartridges saturated with non-radioactive simulant liquid waste as raw material, whose components are equivalent to actual waste. Differences in inherent characteristics of the thermal behavior between the 2nd and the 3rd melter due to the difference in design were considered to establish the procedure to control the new melter. The melter's condition was stabilized at a higher glass temperature and the cooling of 1 kW less in each of the two main electrodes, compared to the 2nd one. Under 39 kW joule heating of the main electrodes with 26 Nm3/h coolant flow rate, it showed the capability to finish heating the bottom furnace in 5 hours before pouring, 2 hours shorter than the 2nd melter. Measurements of the temperature distributions in molten glass and casing surface yielded data that is efficient for developing a simulation model. After Platinum Group Elements (PGE) concentration saturates in the molten glass, feeding raw material and discharging glass were suspended to examine a holding state, indicating PGE settling could retard. During the holding test, observation of the melting process of the cold cap declared that the surface was covered by a thin layer with almost non-fluidity. It will be a reason for choosing the no-slip condition of a fluid calculation, even in the hot-top condition. The investigation of PGE discharging behavior by analyzing the elemental composition of poured glass showed the accumulated PGE amount in the 3rd melter is small compared to the 2nd melter. Inspection of the melter inside after draining out concluded that there were neither significant residual glass nor refractory fragments.
Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:2 Percentile:43.92(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
Otsuki, Daiki*; Ishida, Tatsuhiro*; Tsutsumi, Naoya*; Kobayashi, Masaki*; Inagaki, Kodai*; Yoshida, Teppei*; Takeda, Yukiharu; Fujimori, Shinichi; Yasui, Akira*; Kitagawa, Saiki*; et al.
Physical Review Materials (Internet), 7(12), p.124601_1 - 124601_6, 2023/12
Times Cited Count:2 Percentile:20.33(Materials Science, Multidisciplinary) , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:6 Percentile:84.29(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
Asahi, Yoshimitsu; Shimamura, Keisuke*; Kobayashi, Hidekazu; Kodaka, Akira
JAEA-Technology 2021-026, 50 Pages, 2022/03
In Tokai Reprocessing Plant, the highly active liquid waste derived from a spent fuel reprocessing is vitrified with a Liquid-Fed Ceramic Melter (LFCM) embedded in Tokai Vitrification Facility (TVF). For an LFCM, the viscosity of melted glass is increased by the deposition of oxidation products of platinum group elements (PGE) and the PGE-containing glass tends to settle to the melter's bottom basin even after draining glass out. Removal of the PGE-containing glass is needed to avoid the Joule heating current from being affected by the glass, it requires time-consuming work to remove. For the early accomplishment of vitrifying the waste, Japan Atomic Energy Agency is planning to replace the current melter with the new one in which the amount of PGE sediments would be reduced. In the past design activities for the next melter, several kinds of shapes in regard to the furnace bottom and the strainer were drawn. Among these designs, the one in which the discharge ratio of PGE-containing glass would be as much as or greater than the current melter and which be able to perform similar operational sequences done in the current melter is selected here. Firstly, an operational sequence to produce one canister of vitrified waste is simulated for three melter designs with a furnace bottom shape, using 3D thermal-hydraulic calculations. The computed temperature distribution and its changes are compared among the candidate structures. After discussions about the technical and structural feasibilities of each design, a cone shape with a 45 slope was selected as the bottom shape of the next melter. Secondly, five strainer designs that fit the bottom shape above mentioned are drawn. For each design, the fluid drag and the discharge ratio of relatively high viscosity fluid resting near the bottom are estimated, using steady or unsteady CFD simulation. By draining silicone oil from acrylic furnace models, it was confirmed experimentally that there are no vortices
slope was selected as the bottom shape of the next melter. Secondly, five strainer designs that fit the bottom shape above mentioned are drawn. For each design, the fluid drag and the discharge ratio of relatively high viscosity fluid resting near the bottom are estimated, using steady or unsteady CFD simulation. By draining silicone oil from acrylic furnace models, it was confirmed experimentally that there are no vortices
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.
Komatsu, Yuya*; Shimizu, Ryota*; Wilde, M.*; Kobayashi, Shigeru*; Sasahara, Yuki*; Nishio, Kazunori*; Shigematsu, Kei*; Otomo, Akira*; Fukutani, Katsuyuki; Hitosugi, Taro*
Crystal Growth & Design, 20(9), p.5903 - 5907, 2020/09
Times Cited Count:5 Percentile:45.29(Chemistry, Multidisciplinary) -isosaccharinic acid
-isosaccharinic acidKobayashi, Taishi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Chemical Thermodynamics, 138, p.151 - 158, 2019/11
Times Cited Count:6 Percentile:23.66(Thermodynamics)The effect of  -isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH
-isosaccharinic acid (ISA) on the solubility and redox of tetravalent and hexavalent uranium (U(IV), U(VI)) was investigated in the hydrogen ion concentration (pH ) range of 6
) range of 6 13 and at total ISA concentration ([ISA]
13 and at total ISA concentration ([ISA]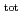 ) = 10
) = 10
 10
10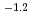 mol/dm
mol/dm . The dependence of U(IV) solubility on pH
. The dependence of U(IV) solubility on pH and [ISA]
and [ISA]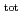 suggested the existence of U(OH)
suggested the existence of U(OH) (ISA)
(ISA)
 as a dominant species within the investigated pH
as a dominant species within the investigated pH range of 6
range of 6 12. For the U(VI)-ISA system, UO
12. For the U(VI)-ISA system, UO (OH)
(OH) (ISA)
(ISA)
 was suggested as a dominant species at pH
was suggested as a dominant species at pH 7
7 13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
13. The formation constants of the U(IV)-ISA and U(VI)-ISA complexes were determined by least-squares fitting of the solubility data. The solubility of U(IV) and U(VI) in the presence of ISA and its effect on the redox behavior were thermodynamically interpreted based on the obtained constants.
 As
As and LiFeAs by X-ray PDF and XAFS analyses
and LiFeAs by X-ray PDF and XAFS analysesLi, S.*; Toyoda, Masayuki*; Kobayashi, Yoshiaki*; Ito, Masayuki*; Ikeuchi, Kazuhiko*; Yoneda, Yasuhiro; Otani, Akira*; Matsumura, Daiju; Asano, Shun*; Mizuki, Junichiro*; et al.
Physica C, 555, p.45 - 53, 2018/12
Times Cited Count:2 Percentile:8.97(Physics, Applied)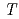 -dependence of local distortions in BaFe
-dependence of local distortions in BaFe As
As and LiFeAs by X-ray PDF and XAFS methods. Although PDF data exhibit anomaly at the structure transition temperature, EXAFS data exhibit no anomaly. Data supporting the local orthorhombicity at 300 K in the tetragonal phase for BaFe
and LiFeAs by X-ray PDF and XAFS methods. Although PDF data exhibit anomaly at the structure transition temperature, EXAFS data exhibit no anomaly. Data supporting the local orthorhombicity at 300 K in the tetragonal phase for BaFe As
As . Arguments on the origins of the 4-fold symmetry breaking in the ground average structure of the tetragonal phase.
. Arguments on the origins of the 4-fold symmetry breaking in the ground average structure of the tetragonal phase.
 addition on water durability of FeO-Fe
addition on water durability of FeO-Fe O
O -P
-P O
O glasses
glassesKitamura, Naoto*; Nomura, Akira*; Saito, Akira*; Kobayashi, Hidekazu; Amamoto, Ippei; Takebe, Hiromichi*
Journal of the Ceramic Society of Japan, 126(11), p.948 - 951, 2018/11
Times Cited Count:6 Percentile:23.20(Materials Science, Ceramics)We studied compositional dependence of water durability of Zr(IV) containing FeO-Fe O
O -P
-P O
O glasses systems, which can apply to immobilize nuclear waste of Zr isotope. Stabilized film with interference fringe on the surface improves better water durability after immersion tests for BaO and ZrO
glasses systems, which can apply to immobilize nuclear waste of Zr isotope. Stabilized film with interference fringe on the surface improves better water durability after immersion tests for BaO and ZrO coexisting glasses without fracture. On the other hand, microcrystalline ZrP
coexisting glasses without fracture. On the other hand, microcrystalline ZrP O
O was detected in the glass matrix when more than 1 mol% of ZrO
was detected in the glass matrix when more than 1 mol% of ZrO incorporated. The effect of impregnated ZrP
incorporated. The effect of impregnated ZrP O
O crystal on the structure was discussed based on the phosphate structure analyzed by Raman spectra. Formation of Q
crystal on the structure was discussed based on the phosphate structure analyzed by Raman spectra. Formation of Q and Q
and Q units, which contribute to water durability in the glass, are due to preferential precipitation of ZrP
units, which contribute to water durability in the glass, are due to preferential precipitation of ZrP O
O crystal.
crystal.
 filament arc-driven multi-cusp ion source for iBNCT
filament arc-driven multi-cusp ion source for iBNCTShibata, Takanori*; Takagi, Akira*; Ikegami, Kiyoshi*; Sugimura, Takashi*; Nammo, Kesao*; Naito, Fujio*; Kobayashi, Hitoshi*; Kurihara, Toshikazu*; Honda, Yosuke*; Sato, Masaharu*; et al.
Proceedings of 15th Annual Meeting of Particle Accelerator Society of Japan (Internet), p.385 - 387, 2018/10
 (am) solubility at 25
(am) solubility at 25 C in the Ca
C in the Ca -Na
-Na -H
-H -Cl
-Cl -OH
-OH -H
-H O system; A Critical review
O system; A Critical reviewRai, D.*; Kitamura, Akira; Altmaier, M.*; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Journal of Solution Chemistry, 47(5), p.855 - 891, 2018/05
Times Cited Count:12 Percentile:10.90(Chemistry, Physical)We have critically reviewed experimental data for Zr hydrolysis constant values for formation of several mononuclear and polynuclear species and a solubility product value for ZrO (am). We have determined new/revised values for the formation constants of Zr(OH)
(am). We have determined new/revised values for the formation constants of Zr(OH)
 , Zr(OH)
, Zr(OH) (aq), Zr(OH)
(aq), Zr(OH)
 , Zr(OH)
, Zr(OH)
 and Ca
and Ca Zr(OH)
Zr(OH)
 , and the solubility product for ZrO
, and the solubility product for ZrO (am) after the critical review.
(am) after the critical review.
Yoshigoe, Akitaka; Shiwaku, Hideaki; Kobayashi, Toru; Shimoyama, Iwao; Matsumura, Daiju; Tsuji, Takuya; Nishihata, Yasuo; Kogure, Toshihiro*; Okochi, Takuo*; Yasui, Akira*; et al.
Applied Physics Letters, 112(2), p.021603_1 - 021603_5, 2018/01
Times Cited Count:7 Percentile:29.91(Physics, Applied)A synchrotron radiation photoemission electron microscope (SR-PEEM) was applied to demonstrate pinpoint analysis of micrometer-sized weathered biotite clay particles with artificially adsorbed cesium (Cs) atoms. Despite the insulating properties of the clay, we observed the spatial distributions of constituent elements (Si, Al, Cs, Mg, Fe) without charging issues. We found that Cs atoms were likely to be adsorbed evenly over the entire particle. Spatially-resolved X-ray absorption spectra (XAS) of the Cs M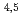 -edge region showed Cs to be present in a monocation state (Cs
-edge region showed Cs to be present in a monocation state (Cs ). Further pinpoint XAS measurements were also performed at the Fe L
). Further pinpoint XAS measurements were also performed at the Fe L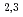 -edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
-edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
Sasaki, Takayuki*; Kokami, Takayuki*; Kobayashi, Taishi*; Kirishima, Akira*; Murakami, Hiroaki; Amano, Yuki; Mizuno, Takashi; Iwatsuki, Teruki; Sasamoto, Hiroshi; Miyakawa, Kazuya
Journal of Nuclear Science and Technology, 54(3), p.373 - 381, 2017/03
Trace amounts of natural thorium and uranium in deep groundwater were investigated at two underground research laboratories situated at Horonobe and Mizunami, Japan. The groundwater was sampled from underground boreholes, and the colloid contribution was checked by in situ two size-fractionated ultrafiltration systems. A decrease in the concentration after in situ filtration suggested the presence of natural colloids and suspended matter that were carriers of a portion of the elements. The result of the Th and U concentrations in groundwater after 10 kDa filtration was analyzed thermodynamically using existing hydrogeological and geochemical data such as the mineral components in the groundwater at a given pH, ionic strength, concentration of co-existing ions, redox potential, and solid phase assumed. A crystalline solid phase made the solubility very low compared with that of the amorphous phase, and the solubility agreed well with the concentrations measured.
Kobayashi, Taishi*; Teshima, Takeshi*; Sasaki, Takayuki*; Kitamura, Akira
Journal of Nuclear Science and Technology, 54(2), p.233 - 241, 2017/02
Times Cited Count:10 Percentile:60.26(Nuclear Science & Technology)Zr solubility in the presence of gluconic acid (GLU) and isosaccharinic acid (ISA) was investigated as a function of hydrogen ion concentration (pH ) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH
) and the total concentration of GLU or ISA. The dependence of the increase in Zr solubility on the pH and GLU concentration suggested the existence of Zr(OH)
and GLU concentration suggested the existence of Zr(OH) (GLU)
(GLU)
 in the neutral pH region and Zr(OH)
in the neutral pH region and Zr(OH) (GLU)(GLU
(GLU)(GLU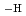 )
) in the alkaline pH region above pH
in the alkaline pH region above pH 10 as the dominant species in the presence of 10
10 as the dominant species in the presence of 10 - 10
- 10 mol/dm
mol/dm (M) GLU. In the presence of ISA, the dominant species Zr(OH)
(M) GLU. In the presence of ISA, the dominant species Zr(OH) (ISA)
(ISA)
 and Zr(OH)
and Zr(OH) (ISA)(ISA
(ISA)(ISA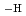 )
) were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH)
were proposed to occur in the neutral and alkaline pH regions, similar to those found in the presence of GLU. From X-ray diffraction analysis, the solubility-limiting solid phase in the presence of GLU and ISA was considered to be Zr(OH) (am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
(am). The formation constants of the Zr gluconate and isosaccharinate complexes were determined by least squares fitting analysis of the solubility data, and the obtained values were discussed in comparison with those of tetravalent actinides.
Naito, Fujio*; Anami, Shozo*; Ikegami, Kiyoshi*; Uota, Masahiko*; Ouchi, Toshikatsu*; Onishi, Takahiro*; Oba, Toshiyuki*; Obina, Takashi*; Kawamura, Masato*; Kumada, Hiroaki*; et al.
Proceedings of 13th Annual Meeting of Particle Accelerator Society of Japan (Internet), p.1244 - 1246, 2016/11
The proton linac installed in the Ibaraki Neutron Medical Research Center is used for production of the intense neutron flux for the Boron Neutron Capture Therapy (BNCT). The linac consists of the 3-MeV RFQ and the 8-MeV DTL. Design average beam current is 10mA. Target is made of Beryllium. First neutron production from the Beryllium target was observed at the end of 2015 with the low intensity beam as a demonstration. After the observation of neutron production, a lot of improvement s was carried out in order to increase the proton beam intensity for the real beam commissioning. The beam commissioning has been started on May 2016. The status of the commissioning is summarized in this report.
 (cr)
(cr)Rai, D.*; Kitamura, Akira; Rosso, K. M.*; Sasaki, Takayuki*; Kobayashi, Taishi*
Radiochimica Acta, 104(8), p.583 - 592, 2016/08
Times Cited Count:6 Percentile:45.07(Chemistry, Inorganic & Nuclear)Solubility studies were conducted with HfO (cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400
(cr) solid as a function of acid concentrations. These studies involved (1) using two different amounts of the solid phase, (2) acid washing the bulk solid phase, (3) preheating the solid phase to 1400  C, and (4) heating amorphous HfO
C, and (4) heating amorphous HfO (am) suspensions to 90
(am) suspensions to 90  C to ascertain whether the HfO
C to ascertain whether the HfO (am) converts to HfO
(am) converts to HfO (cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO
(cr) and to determine the solubility from the oversaturation direction. Based on the results of these treatments it is concluded that the HfO (cr) contains a small fraction of less crystalline, but not amorphous, material [HfO
(cr) contains a small fraction of less crystalline, but not amorphous, material [HfO (lcr)] and this, rather than the HfO
(lcr)] and this, rather than the HfO (cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log
(cr), is the solubility-controlling phase in the range of experimental variables investigated in this study. The solubility data are interpreted using both the Pitzer and SIT models. The log of the solubility product of HfO
of the solubility product of HfO (cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
(cr) is estimated. The observation of a small fraction of less crystalline higher solubility material is consistent with the general picture that mineral surfaces are often structurally and/or imperfect leading to a higher solubility than the bulk crystalline solid. This study stresses the urgent need, during interpretation of solubility data, of taking precautions to make certain that the observed solubility behavior for sparingly-soluble solids is assigned to the proper solid phase.
Tanigawa, Masafumi; Mukai, Yasunobu; Tobita, Hiroshi; Kurata, Noritaka*; Kobayashi, Nozomi*; Takase, Misao*; Makino, Risa; Ozu, Akira; Nakamura, Hironobu; Kurita, Tsutomu; et al.
56th Annual Meeting of the Institute of Nuclear Materials Management (INMM 2015), Vol.1, p.693 - 701, 2016/00
no abstracts in English
 Mo/
Mo/ Tc production by neutron activation method
Tc production by neutron activation methodIshida, Takuya; Shiina, Takayuki*; Ota, Akio*; Kimura, Akihiro; Nishikata, Kaori; Shibata, Akira; Tanase, Masakazu*; Kobayashi, Masaaki*; Sano, Tadafumi*; Fujihara, Yasuyuki*; et al.
JAEA-Technology 2015-030, 42 Pages, 2015/11
The research and development (R&D) on the production of  Mo/
Mo/ Tc by neutron activation method ((n,
Tc by neutron activation method ((n,  ) method) using JMTR has been carried out in the Neutron Irradiation and Testing Reactor Center. The specific radioactivity of
) method) using JMTR has been carried out in the Neutron Irradiation and Testing Reactor Center. The specific radioactivity of  Mo by (n,
Mo by (n,  ) method is extremely low compared with that by fission method ((n,f) method), and as a result, the radioactive concentration of the obtained
) method is extremely low compared with that by fission method ((n,f) method), and as a result, the radioactive concentration of the obtained  Tc solution is also lowered. To solve the problem, we propose the solvent extraction with methyl ethyl ketone (MEK) for recovery of
Tc solution is also lowered. To solve the problem, we propose the solvent extraction with methyl ethyl ketone (MEK) for recovery of  Tc from
Tc from  Mo produced by (n,
Mo produced by (n,  ) method. We have developed the
) method. We have developed the  Mo/
Mo/ Tc separation/extraction/concentration devices and have carried out the performance tests for recovery of
Tc separation/extraction/concentration devices and have carried out the performance tests for recovery of  Tc from
Tc from  Mo produced by (n,
Mo produced by (n,  ) method. In this paper, in order to establish an experimental system for
) method. In this paper, in order to establish an experimental system for  Mo/
Mo/ Tc production, the R&D results of the system are summarized on the improvement of the devices for high-recovery rate of
Tc production, the R&D results of the system are summarized on the improvement of the devices for high-recovery rate of  Tc, on the dissolution of the pellets, which is the high-density molybdenum trioxide (MoO
Tc, on the dissolution of the pellets, which is the high-density molybdenum trioxide (MoO ) pellets irradiated in Kyoto University Research Reactor (KUR), on the production of
) pellets irradiated in Kyoto University Research Reactor (KUR), on the production of  Tc, and on the inspection of the recovered
Tc, and on the inspection of the recovered  Tc solutions.
Tc solutions.
Ozu, Akira; Takase, Misao*; Haruyama, Mitsuo; Kurata, Noritaka*; Kobayashi, Nozomi*; Kureta, Masatoshi; Nakamura, Tatsuya; To, Kentaro; Sakasai, Kaoru; Suzuki, Hiroyuki; et al.
Nuclear Instruments and Methods in Physics Research A, 798, p.62 - 69, 2015/10
Times Cited Count:2 Percentile:16.04(Instruments & Instrumentation)The light transport properties of scintillator light inside alternative He-3 neutron detector modules using scintillator sheets have been investigated by a ray-tracing simulation code. The detector module consists of a light-reflecting tube, a thin rectangular ceramic scintillator sheet laminated on a glass plate, and two photo-multiplier tubes (PMTs) mounted at both ends of the detector tube. The light induced on the surface of the scintillator sheet via nuclear interaction between the scintillator and neutrons are detected by the two PMTs. The light output of various detector modules in which the scintillator sheets are installed with several different arrangements were examined and evaluated in comparison with experimental results. The results derived from the simulation reveal that the light transport property is strongly dependent on the arrangement of the scintillator sheet inside the tube and the shape of the tube.