Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyakawa, Kazuya; Ishii, Eiichi; Imai, Hisashi*; Hirai, Satoru*; Ono, Hirokazu; Nakata, Kotaro*; Hasegawa, Takuma*
Genshiryoku Bakkuendo Kenkyu (CD-ROM), 31(2), p.82 - 95, 2024/12
no abstracts in English
Miyakawa, Kazuya; Hayano, Akira; Sato, Naomi; Nakata, Kotaro*; Hasegawa, Takuma*
JAEA-Data/Code 2023-009, 103 Pages, 2023/09
This borehole investigation was carried out to confirm the validity of the distribution of low flow areas deep underground estimated based on the geophysical survey in FY 2020, as a part of an R&D supporting program titled "Research and development on Groundwater Flow Evaluation Technology in Bedrock" under contract to the Ministry of Economy, Trade and Industry (2021, 2022 FY, Grant Number: JPJ007597). The borehole name is Horonobe Fossil seawater Boring-1 and is referred to as HFB-1 borehole. HFB-1 is a vertical borehole drilled adjacent to the Horonobe Underground Research Laboratory (URL), which was drilled from the surface to a depth of 200 m in FY2021 and from a depth of 200 m to 500 m in FY2022. This report summarizes information related to the drilling of HFB-1 and various data (rock core description, geophysical logging, chemical analysis, etc.) obtained from the borehole investigation.
Miyakawa, Kazuya; Nakata, Kotaro*
JAEA-Data/Code 2022-013, 19 Pages, 2023/03
In the Horonobe Underground Research Laboratory (URL) project, groundwater chemistry was analyzed to investigate changes due to the excavation of the underground facility and to review geochemical models until the fiscal year 2019. From the fiscal year 2020, to proceed remaining important issues deduced from the conclusion of the investigations during the fiscal year 2015-2019, primary data such as groundwater chemistry need to be successively acquired. Here, the chemical analysis of 54 groundwater samples in 2022 from boreholes drilled in the 140 m, 250 m, 350 m gallery in the Horonobe URL, and water rings settled in three vertical shafts is presented. Analytical results include groundwater chemistry such as pH, electrical conductivity, dissolved components (Na , K
, K , Ca
, Ca , Mg
, Mg , Li
, Li , NH
, NH
 , F
, F , Cl
, Cl , Br
, Br , NO
, NO
 , NO
, NO
 , PO
, PO
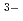 , SO
, SO
 , Total-Mn, Total-Fe, Al, B, Sr, Ba, I, alkalinity, dissolved organic carbon, dissolved inorganic carbon, CO
, Total-Mn, Total-Fe, Al, B, Sr, Ba, I, alkalinity, dissolved organic carbon, dissolved inorganic carbon, CO
 , HCO
, HCO
 , Fe
, Fe , sulfide), and
, sulfide), and 
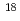 O,
O,  D along with a detailed description of analytical methods.
D along with a detailed description of analytical methods.
Miyakawa, Kazuya; Kashiwaya, Koki*; Komura, Yuto*; Nakata, Kotaro*
Geochemical Journal, 57(5), p.155 - 175, 2023/00
Times Cited Count:2 Percentile:30.17(Geochemistry & Geophysics)In the thick marine sediments, groundwater altered from seawater during the burial diagenesis may exist. Such altered ancient seawater will be called fossil seawater. In such a field, groundwater flow is considered extremely slow because it is not affected by the seepage of meteoric water even after the uplift. During diagenesis, dehydration from silicates causes changes such as a decrease in the salinity of the porewater. However, dehydration reactions alone cannot quantitatively explain water chemistry changes. In this study, we developed an analytical model that considers the dehydration reaction from silicates during the burial process and the upward migration of porewater due to compaction and examined the possible evolution of porewater chemistry. The results showed that the water chemistry, which was strongly influenced by the dehydration reaction from opal-A to quartz and from smectite, was similar to the observations from boring surveys. The results suggest that the fossil seawater formed during the diagenesis may have been preserved since the uplift and strongly supports the slow groundwater flow in the area where the fossil seawater exists.
Nakata, Kotaro*; Hasegawa, Takuma*; Solomon, D. K.*; Miyakawa, Kazuya; Tomioka, Yuichi*; Ota, Tomoko*; Matsumoto, Takuya*; Hama, Katsuhiro; Iwatsuki, Teruki; Ono, Masahiko*; et al.
Applied Geochemistry, 104, p.60 - 70, 2019/05
Times Cited Count:8 Percentile:35.73(Geochemistry & Geophysics)no abstracts in English
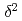 H and
H and 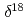 O of water in pores extracted by compression method; Effects of closed pores and comparison to direct vapor equilibration and laser spectrometry method
O of water in pores extracted by compression method; Effects of closed pores and comparison to direct vapor equilibration and laser spectrometry methodNakata, Kotaro*; Hasegawa, Takuma*; Oyama, Takahiro*; Miyakawa, Kazuya
Journal of Hydrology, 561, p.547 - 556, 2018/06
Times Cited Count:5 Percentile:22.07(Engineering, Civil)no abstracts in English
Nakata, Kotaro*; Hasegawa, Takuma*; Oyama, Takahiro*; Ishii, Eiichi; Miyakawa, Kazuya; Sasamoto, Hiroshi
Geofluids, 2018, p.7823195_1 - 7823195_21, 2018/01
Times Cited Count:13 Percentile:63.63(Geochemistry & Geophysics)A groundwater scenario is one of the scenario for safety assessment of geological disposal of high-level radioactive waste. In the safety assessment for groundwater scenario, the slow groundwater flow for a long-term should be an important factor. In the present study, study on stability of groundwater in the Koetoi and Wakkanai formations of Neogene marine based sedimentary rock at the Horonobe area, Hokkaido was performed by investigating the isotopes of chlorine and helium, and the stable isotopes of water. As the results, the stability of groundwater in deeper part of the Wakkanai formation was suggested due to no direct evidence of meteoric water intrusion during the uplift since ca. 1 Ma. Contrary, the groundwater both in the Koetoi formation and the upper Wakkanai formation would be unstable because the meteoric water intrusion was suggested by paleohydrogeological condition and the results of groundwater dating. Likely the Horonobe area, the accurate dating of groundwater would be difficult due to the complex effects of upward and mixing water derived from diagenesis in the thick sediment formation. However, a comparative procedure using both the results of groundwater dating and paleohydrogeological information would be useful for general evaluation of groundwater flow conditions for the long-term (i.e., check the possibility for long-term stability of groundwater).
Miyakawa, Kazuya; Tamamura, Shuji*; Nakata, Kotaro*; Hasegawa, Takuma*
JAEA-Data/Code 2016-021, 60 Pages, 2017/03
The Japan Atomic Energy Agency has been involved in ongoing research in the Horonobe area for the purposes of geoscientific research, and research and development (R&D) on technologies to be used for the geological disposal of high-level radioactive waste. The chemistry of groundwater and dissolved gas from deep boreholes has been obtained since H13 fiscal year for R&D on technologies related to geological characterization. Horonobe Research Institute for the Subsurface Environment (H-RISE) has investigated a resources development on promoting effective use of coal bed buried in Hokkaido including the Horonobe area using microbial communities. The data of dissolved gas from the Horonobe groundwater have also been obtained along with the microbiological research by H-RISE. Central Research Institute of Electric Power Industry (CRIEPI) has conducted R&D on technology of groundwater geochronology which is one of technologies to be used for the geological disposal, and noble gas data from the Horonobe groundwater have been obtained by CRIEPI. This report shows a data set which comprises gas data obtained from the Horonobe underground research project during the period from H13 fiscal year to H27 fiscal year.
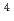 He and
He and  C dating in a granite, Tono area, central Japan
C dating in a granite, Tono area, central JapanHasegawa, Takuma*; Nakata, Kotaro*; Tomioka, Yuichi*; Goto, Kazuyuki*; Kashiwaya, Koki*; Hama, Katsuhiro; Iwatsuki, Teruki; Kunimaru, Takanori*; Takeda, Masaki
Geochimica et Cosmochimica Acta, 192, p.166 - 185, 2016/11
Times Cited Count:10 Percentile:34.66(Geochemistry & Geophysics)Groundwater dating was performed simultaneously by the 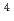 He and
He and  C methods in granite of the Tono area in central Japan. Groundwater was sampled at 30 packed-off sections of six 1000-m boreholes.
C methods in granite of the Tono area in central Japan. Groundwater was sampled at 30 packed-off sections of six 1000-m boreholes. 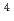 He concentrations increased and
He concentrations increased and  C concentrations decreased along a groundwater flow path on a topographic gradient.
C concentrations decreased along a groundwater flow path on a topographic gradient. 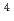 He ages were calculated by using the in situ
He ages were calculated by using the in situ 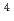 He production rate derived from the porosity, density, and U and Th content of the rock, neglecting external flux. The linear relation between the
He production rate derived from the porosity, density, and U and Th content of the rock, neglecting external flux. The linear relation between the 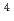 He ages and the noncorrected
He ages and the noncorrected  C ages, except in the discharge area. Simultaneous measurements make it feasible to estimate the accumulation rate of
C ages, except in the discharge area. Simultaneous measurements make it feasible to estimate the accumulation rate of 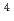 He and initial dilution of
He and initial dilution of  C, which cannot be done with a single method. Cross-checking groundwater dating has the potential to provide more reliable groundwater ages.
C, which cannot be done with a single method. Cross-checking groundwater dating has the potential to provide more reliable groundwater ages.
 C collected by precipitation and gas-strip methods for dating groundwater
C collected by precipitation and gas-strip methods for dating groundwaterNakata, Kotaro*; Hasegawa, Takuma*; Iwatsuki, Teruki; Kato, Toshihiro
Radiocarbon, 58(3), p.491 - 503, 2016/09
Times Cited Count:6 Percentile:22.56(Geochemistry & Geophysics)Dissolved inorganic carbon (DIC) for  C analysis of groundwater is usually extracted by a gas-strip or precipitation method. In this study, the certainty of the two methods for
C analysis of groundwater is usually extracted by a gas-strip or precipitation method. In this study, the certainty of the two methods for  C dating were confirmed. DIC and
C dating were confirmed. DIC and  C concentrations obtained by the gas-strip method were close to the theoretically predicted
C concentrations obtained by the gas-strip method were close to the theoretically predicted  C value. Conversely, the
C value. Conversely, the  C value obtained by the precipitation method always showed higher values than the predicted values. The difference in
C value obtained by the precipitation method always showed higher values than the predicted values. The difference in  C value between gas-strip and precipitation methods was assumed to arise owing to contamination of modern carbon used in the precipitation method. The applicability of the precipitation method for groundwater should be considered carefully according to the DIC,
C value between gas-strip and precipitation methods was assumed to arise owing to contamination of modern carbon used in the precipitation method. The applicability of the precipitation method for groundwater should be considered carefully according to the DIC,  C concentration of groundwater and purpose of the study being conducted.
C concentration of groundwater and purpose of the study being conducted.
Yokoyama, Shingo*; Nakata, Kotaro*; Suzuki, Shinichi
Nendo Kagaku, 54(1), p.28 - 35, 2015/08
no abstracts in English
 H, CFCs and SF
H, CFCs and SF as trace materials
as trace materialsHagiwara, Hiroki; Iwatsuki, Teruki; Hasegawa, Takuma*; Nakata, Kotaro*; Tomioka, Yuichi*
Nihon Suimon Kagakkai-Shi, 45(2), p.21 - 38, 2015/07
This study evaluates a method to estimate shallow groundwater intrusion in and around a large underground research facility (Mizunami Underground Research Laboratory - MIU). Water chemistry, stable isotopes ( D and
D and 
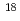 O), tritium (
O), tritium ( H), chlorofluorocarbons (CFCs) and sulfur hexafluoride (SF
H), chlorofluorocarbons (CFCs) and sulfur hexafluoride (SF ) in groundwater were monitored around the facility (from 20 m down to a depth of 500 m), for a period of 5 years. The results show that shallow groundwater inflows into deeper groundwater at depths of between 200-400 m. In addition, the content of shallow groundwater estimated using
) in groundwater were monitored around the facility (from 20 m down to a depth of 500 m), for a period of 5 years. The results show that shallow groundwater inflows into deeper groundwater at depths of between 200-400 m. In addition, the content of shallow groundwater estimated using  H and CFC-12 concentrations is up to a maximum of about 50%. This is interpreted as the impact on the groundwater environment caused by construction and operation of a large facility over several years. The concomitant use of
H and CFC-12 concentrations is up to a maximum of about 50%. This is interpreted as the impact on the groundwater environment caused by construction and operation of a large facility over several years. The concomitant use of  H and CFCs is an effective method to determine the extent of shallow groundwater inflow caused by construction of an underground facility.
H and CFCs is an effective method to determine the extent of shallow groundwater inflow caused by construction of an underground facility.
Ito, Kenichi*; Miyahara, Hidetaka*; Ujiie, Toru*; Takeshima, Toshikatsu*; Yokoyama, Shingo*; Nakata, Kotaro*; Nagano, Tetsushi; Sato, Tsutomu*; Hatta, Tamao*; Yamada, Hirohisa*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 11(4), p.255 - 271, 2012/04
no abstracts in English
Kitamura, Akira; Nakata, Kotaro*; Tanaka, Satoru*; Tomura, Tsutomu*; Kamei, Gento
Saikuru Kiko Giho, (22), p.59 - 66, 2004/03
Redox reactions between neptunium(V) (Np(V)) and magnetite (Fe(II)1Fe(III)2O4) surface were investigated in N gas atmosphere. A batch method was applied to the experiment. High-pure magnetite and a 0.1 M NaCl were mixed in a polypropylene tube, and pH, redox potential and concentration of dissolved neptunium were measured as a function of shaking time (from 1 hour to 7 days), temperature (298 K and 318 K) and liquid/solid ratio (20, 50 and 100 ml.g
gas atmosphere. A batch method was applied to the experiment. High-pure magnetite and a 0.1 M NaCl were mixed in a polypropylene tube, and pH, redox potential and concentration of dissolved neptunium were measured as a function of shaking time (from 1 hour to 7 days), temperature (298 K and 318 K) and liquid/solid ratio (20, 50 and 100 ml.g ). It was observed that the concentration of dissolved neptunium was reduced rapidly within a day, due to the reducing of Np(V) to Np(IV) and the precipitation of Np(IV). This result was shown typically when the magnetite/solution ratio and the temperature were high. The rate constant of the redox reaction and the activation energy for the rate constant were preliminarily obtained. It was suggested that the redox reaction was promoted by not only Fe(II) on magnetite surface but also Fe(II) inside the magnetite.
). It was observed that the concentration of dissolved neptunium was reduced rapidly within a day, due to the reducing of Np(V) to Np(IV) and the precipitation of Np(IV). This result was shown typically when the magnetite/solution ratio and the temperature were high. The rate constant of the redox reaction and the activation energy for the rate constant were preliminarily obtained. It was suggested that the redox reaction was promoted by not only Fe(II) on magnetite surface but also Fe(II) inside the magnetite.
Tanaka, Satoru*; Nagasaki, Shinya*; Nakata, Kotaro*; Oda, Takuji*; Kameda, Jun*; Kamei, Gento; Tachi, Yukio
JNC TY8400 2003-008, 88 Pages, 2003/05
Redox reactions between Cr(VI) and iron(II) chloride (FeCl2) and those between Cr(VI) and magnetite (Fe(II)1Fe(III)2O4) were observed as a preliminary study. According to the experimental results, it was suggested that the redox reactions were promoted more than the amount of Fe(II) on magnetite surface because of electron transfer from internal Fe(II) to magnetite surface. The results were quantitatively supported from quantum chemical calculations. Redox reactions between Np(V) and magnetite and the reduction of Np to tetravalent were observed, while those between Np(V) and FeCl2 were not observed obviously. It was observed that the reactions were promoted rapidly when the magnetite / solution ratio and the temperature were high, and the rate constant of the reactions was obtained. Furthermore, it was found that hydrogen gas and hydrogen ion were generated with crushing the quartz in an inert gas atmosphere.
Nakata, Kotaro*; Nagasaki, Shinya*; Tanaka, Satoru*; Sakamoto, Yoshiaki; Tanaka, Tadao; Ogawa, Hiromichi
Radiochimica Acta, 90(9-11), p.665 - 669, 2002/12
Times Cited Count:53 Percentile:93.51(Chemistry, Inorganic & Nuclear)Sorption and desorption experiments of Np on magnetite and hematite under aerobic and anaerobic conditions were carried out to investigate the possibility of reduction of Np(V) to Np(IV) on the surfave of iron oxides including Fe(II). The results indicated that Np sorption mechanism on magnetite under anaerobic condition was completely different from that under aerobic condition. The evidence of the presence of Np(IV) on magnetite surface after sorption was obtained from the extraction experiment with TTA/xylene solution.
Nakata, Kotaro*; Nagasaki, Shinya*; Tanaka, Satoru*; Sakamoto, Yoshiaki; Tanaka, Tadao; Ogawa, Hiromichi
JAERI-Conf 2002-004, p.667 - 673, 2002/03
no abstracts in English
Nakata, Kotaro*; Nagasaki, Shinya*; Tanaka, Satoru*; Sakamoto, Yoshiaki; Tanaka, Tadao; Ogawa, Hiromichi
Radiochimica Acta, 88(8), p.453 - 457, 2000/12
Times Cited Count:13 Percentile:63.90(Chemistry, Inorganic & Nuclear)Sorption kinetics of Np(V) on magnetite and hematite were investigated, and a sequential desorption method was applied to investigate changes in the chemical form of Np sorbed according to the amount of time they were in contact with the Np solution. It was found that the sorption process consists of fast sorption and slow sorption which reaches equilibrium in 1 h. From the results of sorption and desorption kinetics, it was concluded that the equilibrium between various chemical forms of sorbed Np was achieved in about 1 week, although the amount of sorbed Np reached an equilibrium in only 1 h.
Hama, Katsuhiro; Iwatsuki, Teruki; Nakata, Kotaro*; Kodama, Hiroki*; Hasegawa, Takuma*
no journal, ,
Groundwater residence time can be one of the useful tools to understand regional groundwater flow. The trial groundwater dating has been made using radiocarbon ( C) in the organics that dissolved in groundwater.
C) in the organics that dissolved in groundwater.
Hasegawa, Takuma*; Nakata, Kotaro*; Tomioka, Yuichi*; Goto, Kazuyuki*; Kashiwaya, Koki*; Hama, Katsuhiro
no journal, ,
The groundwater flow velocity is one of the important items in the safety assessment for the HLW disposal. Generally, it is too difficult to measure the groundwater flow velocity directly. Therefore, to determine the groundwater age by radio active isotopes are effective. However, there are some problems to apply the metrology to the natural samples. For example, because the ratio of Carbon isotope changes by dissolving of the carbonate mineral and resolving the organic materials, the development of the technique for correcting this is needed. In this study, the groundwater ages are measured by 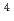 He and
He and  C, etc. and compared these results as a joint research with Central Research Institute of Electric Power Industry.
C, etc. and compared these results as a joint research with Central Research Institute of Electric Power Industry.