Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Iriya, Keishiro*; Fujii, Kensuke*; Kubo, Hiroshi*
JNC TJ8400 2003-068, 144 Pages, 2003/02
None
Iriya, Keishiro*; Fujii, Kensuke*; Kubo, Hiroshi*
JNC TJ8400 2003-067, 285 Pages, 2003/02
Hydraulic conductivities of bentonite and rock under hyper alkaline and nitrate conditions were investigated.
Iriya, Keishiro*; Fujii, Kensuke*; Tajima, Takatoshi*; Takeda, Nobufumi*; Kubo, Hiroshi*
JNC TJ8400 2003-063, 49 Pages, 2003/02
In this study, applicability of low alkalinity cement and alteration of bentonite in the cement ere investigated.
Iriya, Keishiro*; Fujii, Kensuke*; Tajima, Takatoshi*; Takeda, Nobufumi*; Kubo, Hiroshi*
JNC TJ8400 2003-062, 110 Pages, 2003/02
In this study, applicability of low alkalinity cement and alteration of bentonite in the cement ere investigated.
Iriya, Keishiro*; *; Kubo, Hiroshi*
JNC TJ8400 2002-045, 124 Pages, 2002/02
no abstracts in English
Iriya, Keishiro*; *; Kubo, Hiroshi*
JNC TJ8400 2002-044, 260 Pages, 2002/02
The chemical conditions of TRU waste repository were estimated as alkaline conditions effected by cementitios materials. And, some TRU wastes include soluble nitrate salt, we have to consider the repository conditions might be high ionic strength condition leaching of nitrate salt. In this study, experimental studies were carried out to evaluate hydraulic conductivities of bentonite and rock under hyper alkaline and nitrate conditions. The followings results were obtained for bentonite (1)In the immersion experiments of bentonite in hyper alkaline fluids with and without nitrate, the disappearance of montmorillonite of bentontnite was observed and CSH formation was found after 30days. In hyper alkaline fluid with nitrate, minerals at 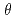 = 37nm by XRD was identified. (2)Significant effects of hyper alkaline on hydraulic conductivity of compacted bentonite were not observed. However, hydraulic conductivities of hyper alkaline fluid with nitrate and ion exchanged bentonite increased. In hyper alkaline with nitrate, more higher hydraulic conductivities of exchanged bentonite were measured. The followings results were obtained for rock. (1) In the immersion experiments of crushed tuff in hyper alkaline fluids with and without nitrate, CSH and CASH phases were observed. (2)The hydraulic conductivity of tuff in hyper alkaline fluids decreased gradually. Finally, hyper alkaline flow in tuff stopped after 2 months and hyper alkaline flow with nitrate stopped shorter than without nitrate. In the results of analysis of tuff after experiment, we could identified secondary minerals, but we couldn't find the clogging evidence of pores in tuff by secondary minerals.
= 37nm by XRD was identified. (2)Significant effects of hyper alkaline on hydraulic conductivity of compacted bentonite were not observed. However, hydraulic conductivities of hyper alkaline fluid with nitrate and ion exchanged bentonite increased. In hyper alkaline with nitrate, more higher hydraulic conductivities of exchanged bentonite were measured. The followings results were obtained for rock. (1) In the immersion experiments of crushed tuff in hyper alkaline fluids with and without nitrate, CSH and CASH phases were observed. (2)The hydraulic conductivity of tuff in hyper alkaline fluids decreased gradually. Finally, hyper alkaline flow in tuff stopped after 2 months and hyper alkaline flow with nitrate stopped shorter than without nitrate. In the results of analysis of tuff after experiment, we could identified secondary minerals, but we couldn't find the clogging evidence of pores in tuff by secondary minerals.
*; *; Kubo, Hiroshi*; Uegaki, Yoshiaki*
JNC TJ8400 2002-039, 38 Pages, 2002/02
A concept of radioactive waste repository in which both bentonite and cementitious materials exist in deep cavern as engineered barriers is proposed. It is pointed out that pore water of cement is approximately 12.0 to 13.0 of pH and that it maintains for a long period. Therefore alteration of bentonite and rocks should be studied. Mixing test upon some interaction between modeled cement water and bentonite and rocks have been carried out since 1995 as a part of TRU repository's study. And low alkalinity of cement has been studied as parallel to study on alteration of bentnite. HFSC which has high fly ash content and which shows approximately 10.5 to 11.0 of pH of pore water was developed. Cementitious materials are generally use as a combination with steel, since its tensile strength is low. The corrosion of steel in concrete becomes a big problem in case of decreasing pH of cement. There is little available reference, since low alkalinity cement is quite new and special ordered one. Accelerating test for corrosion in low alkalinity concrete were carried out in order to collect data of corrosion. Although alteration of bentonite by several types of modeled cement water was tested. Long term test by actual cement pore water has not carried out. The alteration in 360 days was investigated. Conclusion obtained in this study is following. [Corrosion of steel (re-bar)] (1)Re-bar in HFSC with 60% of W/C is significantly corroded. The corrosion rate is bigger than the rate of ordinary used cement. (2)Diffusivity of Cl ion in HFSC is similar to it in OPC comparing by the same water powder ratio. (3)Corrosion rate of HFSC 30 is similar to OPC60. However corrosion is progressed in HFSC 30 without Cl
ion in HFSC is similar to it in OPC comparing by the same water powder ratio. (3)Corrosion rate of HFSC 30 is similar to OPC60. However corrosion is progressed in HFSC 30 without Cl ion due to lower alkalinity, but it isn't done in OPC within a certain amount of Cl
ion due to lower alkalinity, but it isn't done in OPC within a certain amount of Cl ion. [Alteration of bentonite and rocks] (1)Although no secondary minerals was observed in HFSC, monmorironite is gradually lost by increasing calcite. (2)Secondary ...
ion. [Alteration of bentonite and rocks] (1)Although no secondary minerals was observed in HFSC, monmorironite is gradually lost by increasing calcite. (2)Secondary ...
Iriya, Keishiro*; *; Kubo, Hiroshi*; Uegaki, Yoshiaki*
JNC TJ8400 2002-038, 83 Pages, 2002/02
A concept of radioactive waste repository in which both bentonite and cementitious materials exist in deep cavern as engineered barriers is proposed. It is pointed out that pore water of cement is approximately 12.0 to 13.0 of pH and that it maintains for a long period. Therefore alteration of bentonite and rocks should be studied. Mixing test upon some interaction between modeled cement water and bentonite and rocks have been carried out since 1995 as a part of TRU repository's study. And low alkalinity of cement has been studied as parallel to study on alteration of bentnite. HFSC which has high fly ash content and which shows approximately 10.5 to 11.0 of pH of pore water was developed. Cementitious materials are generally use as a combination with steel, since its tensile strength is low. The corrosion of steel in concrete becomes a big problem in case of decreasing pH of cement. There is little available reference, since low alkalinity cement is quite new and special ordered one. Accelerating test for corrosion in low alkalinity concrete were carried out in order to collect data of corrosion. Although alteration of bentonite by several types of modeled cement water was tested. Long term test by actual cement pore water has not carried out. The alteration in 360 days was investigated. Conclusion obtained in this study is following. [Corrosion of steel (re-bar)] (1)Re-bar in HFSC with 60% of W/C is signmcantly corroded. The corrosion rate is bigger than the rate of ordinary used cement. (2)Diffusivity of Cl ion in HFSC is similar to it in OPC comparing by the same water powder ratio. (3)Corrosion rate of HFSC 30 is similar to OPC60. However corrosion is progressed in HFSC 30 without Cl
ion in HFSC is similar to it in OPC comparing by the same water powder ratio. (3)Corrosion rate of HFSC 30 is similar to OPC60. However corrosion is progressed in HFSC 30 without Cl ion due to lower alkalinity, but it isn't done in OPC within a certain amount of Cl
ion due to lower alkalinity, but it isn't done in OPC within a certain amount of Cl ion. [Alteration of bentonite and rocks] (1)Although no secondary minerals was observed in HFSC, monmorironite is gradually lost by increasing calcite. (2)Secondary ...
ion. [Alteration of bentonite and rocks] (1)Although no secondary minerals was observed in HFSC, monmorironite is gradually lost by increasing calcite. (2)Secondary ...