Crystallization and preliminary neutron analysis of the dissimilatory sulfite reductase D (DsrD) protein from the sulfate-reducing bacterium 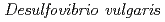
Dissmilatory sulfite reductase Dの結晶化と中性子回折実験
茶竹 俊行; 水野 伸宏*; Voordown, G.*; 樋口 芳樹*; 新井 栄揮; 田中 伊知朗; 新村 信雄
Chatake, Toshiyuki; Mizuno, Nobuhiro*; Voordown, G.*; Higuchi, Yoshiki*; Arai, Shigeki; Tanaka, Ichiro; Niimura, Nobuo
硫酸還元菌(Desulfovibrio vulgaris)由来DsrDは立体構造中にDNA結合モチーフを持つことが判明している。本研究では、DNA認識における水素結合様式及び水和構造ネットワークの解明を目的として、中性子結晶構造解析を試みている。中性子回折実験に必要な大型結晶(1mm 以上)を得るために、結晶析出相図とX線回折実験による結晶化情報の検討と、マクロシーディングを行い、1.7mm
以上)を得るために、結晶析出相図とX線回折実験による結晶化情報の検討と、マクロシーディングを行い、1.7mm の単結晶を得ることに成功した。現在、結晶1軸方向での中性子回折実験データ収集が終わり、構造解析で水素水和構造の概要が見えたので報告する。
の単結晶を得ることに成功した。現在、結晶1軸方向での中性子回折実験データ収集が終わり、構造解析で水素水和構造の概要が見えたので報告する。
Issimilatory sulfite reductase D (DsrD) from 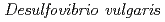 has been crystallized for a neutron diffraction study. The initial crystals obtained were too small for the neutron experiment. In order to obtain a larger crystal (1 mm
has been crystallized for a neutron diffraction study. The initial crystals obtained were too small for the neutron experiment. In order to obtain a larger crystal (1 mm ), a combination of two techniques was used to find the optimum crystallization conditions: a crystallization phase diagram, followed by crystal-quality assessment via X-ray diffraction. Using conditions determined in this manner, a large single crystal (1.7 mm
), a combination of two techniques was used to find the optimum crystallization conditions: a crystallization phase diagram, followed by crystal-quality assessment via X-ray diffraction. Using conditions determined in this manner, a large single crystal (1.7 mm ) of the DsrD protein was subsequently grown in D
) of the DsrD protein was subsequently grown in D O solution by the macro-seeding technique. The neutron diffraction experiment was carried out using the BIX-3 diffractometer at the Japan Atomic Energy Research Institute (JAERI), and the diffraction data up to 2.4 AA resolution could be collected from this crystal.
O solution by the macro-seeding technique. The neutron diffraction experiment was carried out using the BIX-3 diffractometer at the Japan Atomic Energy Research Institute (JAERI), and the diffraction data up to 2.4 AA resolution could be collected from this crystal.