Oxygen potentials of (U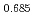 ,Pu
,Pu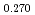 ,Am
,Am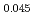 )O
)O solid solutions
solid solutions
None
逢坂 正彦 
 ; 佐藤 勇; 滑川 卓志; 黒崎 健*; 山中 伸介*
; 佐藤 勇; 滑川 卓志; 黒崎 健*; 山中 伸介*
Osaka, Masahiko; Sato, Isamu; Namekawa, Takashi; Kurosaki, Ken*; Yamanaka, Shinsuke*
Amを含有するMOX燃料の酸素ポテンシャルを測定した。H O/H
O/H , CO
, CO /H
/H 気相平衡を用いた熱重量分析により、1123K, 1273K及び1423Kおける酸素ポテンシャルを測定した。測定値をMOX燃料と比べた場合、わずか4.5%のAmであっても酸素ポテンシャルは非常に高いことが分かった。
気相平衡を用いた熱重量分析により、1123K, 1273K及び1423Kおける酸素ポテンシャルを測定した。測定値をMOX燃料と比べた場合、わずか4.5%のAmであっても酸素ポテンシャルは非常に高いことが分かった。
Oxygen potentials of (U,Pu)O containing AmO
containing AmO , (U
, (U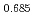 Pu
Pu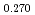 Am
Am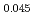 )O
)O , were measured as functions of the oxygen to metal (O/M) ratios and temperatures by hermogravimetric analysis (TGA) and discussed from thermodynamic viewpoints. The (U
, were measured as functions of the oxygen to metal (O/M) ratios and temperatures by hermogravimetric analysis (TGA) and discussed from thermodynamic viewpoints. The (U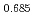 Pu
Pu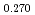 Am
Am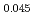 )O
)O solid solution was prepared by a traditional powder metallurgical route. Oxygen partial pressure (pO
solid solution was prepared by a traditional powder metallurgical route. Oxygen partial pressure (pO ) was adjusted in the region from 10
) was adjusted in the region from 10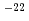 to 10
to 10 MPa by using CO
MPa by using CO /H
/H and H
and H O /H
O /H equilibria. Changes of microgram order in the specimen weight were continuously monitored with a TGA apparatus and the dependences of oxygen potentials on the O/M ratios were obtained at 1123K, 1273K and 1423K. The oxygen potentials of (U
equilibria. Changes of microgram order in the specimen weight were continuously monitored with a TGA apparatus and the dependences of oxygen potentials on the O/M ratios were obtained at 1123K, 1273K and 1423K. The oxygen potentials of (U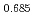 Pu
Pu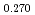 Am
Am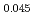 )O
)O were significantly higher than those in (U
were significantly higher than those in (U Pu
Pu )O
)O . The partial molar entropy and enthalpy of oxygen have been calculated and compared with those of Am O
. The partial molar entropy and enthalpy of oxygen have been calculated and compared with those of Am O , (Am
, (Am U
U ) O
) O , and (U
, and (U Pu
Pu )O
)O . Finally, the oxygen potential isotherms of (U
. Finally, the oxygen potential isotherms of (U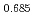 ,Pu
,Pu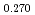 ,Am
,Am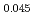 )O
)O were examined in terms of defect structure.
were examined in terms of defect structure.