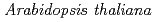 Y-family DNA polymerase
Y-family DNA polymerase 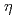 catalyses translesion synthesis and interacts functionally with PCNA2
catalyses translesion synthesis and interacts functionally with PCNA2
シロイヌナズナのYファミリーDNAポリメラーゼ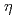 は損傷乗り越え複製を触媒し、PCNA2と機能的に相互作用する
は損傷乗り越え複製を触媒し、PCNA2と機能的に相互作用する
Anderson, H.*; Vonarx, E.*; Pastushok, L.*; 中川 繭; 片渕 淳*; Gruz, P.*; Rubbo, A.*; Grice, D.*; Osmond, M.*; 坂本 綾子; 能美 健彦*; Xiao, W.*; Kunz, B.*
Anderson, H.*; Vonarx, E.*; Pastushok, L.*; Nakagawa, Mayu; Katafuchi, Atsushi*; Gruz, P.*; Rubbo, A.*; Grice, D.*; Osmond, M.*; Sakamoto, Ayako; Nomi, Takehiko*; Xiao, W.*; Kunz, B.*
We assessed the roles of 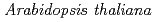
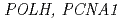 and
and 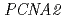 in TLS mediated UV resistance.
in TLS mediated UV resistance. 
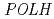 defective mutants sensitized growth of roots and whole plants to UV radiation indicating AtPol
defective mutants sensitized growth of roots and whole plants to UV radiation indicating AtPol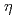 contributes to UV resistance.
contributes to UV resistance. 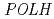 alone did not complement the UV sensitivity conferred by deletion of yeast
alone did not complement the UV sensitivity conferred by deletion of yeast 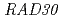 , although AtPol
, although AtPol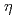 exhibited cyclobutane dimer bypass activity and interacted with yeast PCNA in vitro. Co-expression of
exhibited cyclobutane dimer bypass activity and interacted with yeast PCNA in vitro. Co-expression of 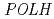 and
and 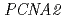 , but not
, but not 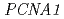 , restored normal UV resistance and mutation kinetics in the
, restored normal UV resistance and mutation kinetics in the 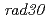 mutant. A single residue difference at site 201, which lies adjacent to the lysine ubiquitylated in PCNA, appeared responsible for the inability of PCNA1 to function with AtPol
mutant. A single residue difference at site 201, which lies adjacent to the lysine ubiquitylated in PCNA, appeared responsible for the inability of PCNA1 to function with AtPol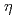 in UV treated yeast. PCNA interacting protein boxes and an ubiquitin-binding motif in AtPol
in UV treated yeast. PCNA interacting protein boxes and an ubiquitin-binding motif in AtPol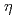 were found to be required for restoration of UV resistance in the
were found to be required for restoration of UV resistance in the 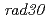 mutant by
mutant by 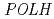 and
and 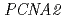 . These observations indicate AtPol
. These observations indicate AtPol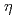 can catalyse TLS past UV induced DNA damage, and link the biological activity of AtPol
can catalyse TLS past UV induced DNA damage, and link the biological activity of AtPol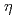 in UV irradiated cells to PCNA2 and PCNA-and ubiquitin-binding motifs in AtPol
in UV irradiated cells to PCNA2 and PCNA-and ubiquitin-binding motifs in AtPol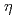 .
.