Discovery of proteinaceous N-modification in lysine biosynthesis of 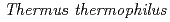
高度高熱菌リジン合成系ではタンパク質がN末を改変している
堀江 暁*; 富田 武郎*; 斉木 朝子*; 河野 秀俊; 高 ひかり*; 峯木 礼子*; 藤村 務*; 西山 千春*; 葛山 智久*; 西山 真*
Horie, Akira*; Tomita, Takeo*; Saiki, Asako*; Kono, Hidetoshi; Taka, Hikari*; Mineki, Reiko*; Fujimura, Tsutomu*; Nishiyama, Chiharu*; Kuzuyama, Tomohisa*; Nishiyama, Makoto*
Although the latter part of the lysine biosynthesis, conversion of  -aminoadipate (AAA) to lysine, of
-aminoadipate (AAA) to lysine, of 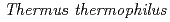 is similar to that of the arginine biosynthesis, enzymes homologous to ArgA and ArgJ are absent in the Thermus pathway. Since ArgA and ArgJ are known to modify the amino group of glutamate to avoid intramolecular cyclization of intermediates, their absence suggests an alternative N-modification system in the pathway. We reconstituted the conversion of AAA to lysine, and revealed that the amino group of AAA is modified with the
is similar to that of the arginine biosynthesis, enzymes homologous to ArgA and ArgJ are absent in the Thermus pathway. Since ArgA and ArgJ are known to modify the amino group of glutamate to avoid intramolecular cyclization of intermediates, their absence suggests an alternative N-modification system in the pathway. We reconstituted the conversion of AAA to lysine, and revealed that the amino group of AAA is modified with the  -carboxyl group of C-terminal Glu54 of a small protein, LysW, and the side-chain of AAA is converted to the lysyl side-chain in LysW-attached forms. Lysine is subsequently liberated from LysW/lysine-fusion. Recognition of the acidic globular domain of LysW by biosynthetic enzymes indicates that LysW acts as a carrier protein or protein scaffold of the biosynthetic enzymes in this system. This study reveals the previously unknown function of a small protein in the primary metabolism.
-carboxyl group of C-terminal Glu54 of a small protein, LysW, and the side-chain of AAA is converted to the lysyl side-chain in LysW-attached forms. Lysine is subsequently liberated from LysW/lysine-fusion. Recognition of the acidic globular domain of LysW by biosynthetic enzymes indicates that LysW acts as a carrier protein or protein scaffold of the biosynthetic enzymes in this system. This study reveals the previously unknown function of a small protein in the primary metabolism.