Purification, crystallization and preliminary X-ray diffraction analysis of DNA damage response A protein from 
 DNA損傷応答タンパク質Aの精製,結晶化及びX線回折分析
DNA損傷応答タンパク質Aの精製,結晶化及びX線回折分析
山田 貢; 佐藤 勝也; 鳴海 一成
Yamada, Mitsugu; Sato, Katsuya; Narumi, Issei
放射線抵抗性細菌 由来のDNA損傷応答タンパク質(DdrA)は、一本鎖DNAの3'末端に結合し、DNA分解酵素からDNA末端を保護することで、DNA修復過程に関与していると考えられている。本研究では、タンパク質構造情報に基づいてDdrAタンパク質の生理的役割を明らかにする目的で、N末端から157残基のアミノ酸配列からなるC末端欠損DdrAタンパク質の結晶を作製し、KEK-PFのBL5ビームラインにてX線結晶回折実験を行った。その結果、最高2.35
由来のDNA損傷応答タンパク質(DdrA)は、一本鎖DNAの3'末端に結合し、DNA分解酵素からDNA末端を保護することで、DNA修復過程に関与していると考えられている。本研究では、タンパク質構造情報に基づいてDdrAタンパク質の生理的役割を明らかにする目的で、N末端から157残基のアミノ酸配列からなるC末端欠損DdrAタンパク質の結晶を作製し、KEK-PFのBL5ビームラインにてX線結晶回折実験を行った。その結果、最高2.35 の分解能の回折像を得ることに成功した。結晶は、擬欠面双晶の非対称ユニット中に14分子を含んでおり、7量体リングが2つ重なった高次構造を有していると考えられた。
の分解能の回折像を得ることに成功した。結晶は、擬欠面双晶の非対称ユニット中に14分子を含んでおり、7量体リングが2つ重なった高次構造を有していると考えられた。
DNA damage response A protein (DdrA) from  has been suggested to be involved in DNA repair processes through binding to 3' ends of single-stranded DNA, thereby protecting the ends from nuclease digestion. In this study, a recombinant C-terminal truncated form of
has been suggested to be involved in DNA repair processes through binding to 3' ends of single-stranded DNA, thereby protecting the ends from nuclease digestion. In this study, a recombinant C-terminal truncated form of 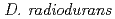 DdrA (DdrA157), comprising of the first 157 residues of DdrA, was expressed in
DdrA (DdrA157), comprising of the first 157 residues of DdrA, was expressed in 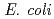 , purified and crystallized. Single crystals of DdrA157 were obtained by a hanging drop method at 293 K. The crystal belonged to the monoclinic space group
, purified and crystallized. Single crystals of DdrA157 were obtained by a hanging drop method at 293 K. The crystal belonged to the monoclinic space group  2
2 , with unit-cell parameters
, with unit-cell parameters  = 46.31,
= 46.31,  = 180.26,
= 180.26,  = 114.17
= 114.17  ,
, 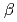 = 90.02
= 90.02 . The crystal was expected to contain fourteen molecules in the asymmetric unit. Diffraction data were collected to 2.35
. The crystal was expected to contain fourteen molecules in the asymmetric unit. Diffraction data were collected to 2.35  resolution at beamline BL-5 of the Photon Factory and initial phase determinations were attempted by a molecular-replacement method using the human Rad52 structure.
resolution at beamline BL-5 of the Photon Factory and initial phase determinations were attempted by a molecular-replacement method using the human Rad52 structure.