Medical application of radiohalogenated peptides; Synthesis and 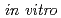 evaluation of F(
evaluation of F(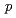 -
- I)KCCYSL for targeting HER2
I)KCCYSL for targeting HER2
放射性ハロゲン標識ペプチドの医学的応用; HER2を標的としたF(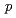 -
- I)KCCYSLの合成と
I)KCCYSLの合成と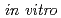 評価
評価
佐々木 一郎; 渡辺 茂樹; 大島 康宏; 須郷 由美; 山田 圭一*; 花岡 宏史*; 石岡 典子
Sasaki, Ichiro; Watanabe, Shigeki; Ohshima, Yasuhiro; Sugo, Yumi; Yamada, Keiichi*; Hanaoka, Hirofumi*; Ishioka, Noriko
Radioisotope labeled peptides with high affinity to receptors overexpressing on the surface of tumor cells are promising for applications in nuclear medicine such as diagnostic radiography and radiotherapy. Radiohalogens such as  I and
I and  At are useful for clinical imaging and therapeutic applications, and it can be introduced at the
At are useful for clinical imaging and therapeutic applications, and it can be introduced at the 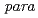 position of phenylalanine residue via electrophilic destannylation. KCCYSL (Lys
position of phenylalanine residue via electrophilic destannylation. KCCYSL (Lys -Cys
-Cys -Cys
-Cys -Tyr
-Tyr -Ser
-Ser -Leu
-Leu ) is a hexapeptide containing disulfide bond. Previous study revealed that KCCYSL has potential as tumor imaging and therapeutic agent targeting tumor cells overexpressing the human epidermal growth factor receptor type 2 (HER2). In this study, we report synthesis and
) is a hexapeptide containing disulfide bond. Previous study revealed that KCCYSL has potential as tumor imaging and therapeutic agent targeting tumor cells overexpressing the human epidermal growth factor receptor type 2 (HER2). In this study, we report synthesis and 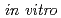 evaluation of radiohalogenated KCCYSL derivatives. Precursor peptides, Boc-F(
evaluation of radiohalogenated KCCYSL derivatives. Precursor peptides, Boc-F(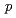 -SnBu
-SnBu )K(Boc)C(Trt)C(Trt)Y(
)K(Boc)C(Trt)C(Trt)Y(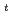 Bu)S(
Bu)S(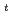 Bu)L-OH and Boc-F(
Bu)L-OH and Boc-F(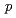 -SnBu
-SnBu )GS(
)GS(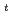 Bu)GK(Boc)C(Trt)C(Trt)Y(
Bu)GK(Boc)C(Trt)C(Trt)Y(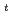 Bu)S(
Bu)S(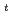 Bu)L-OH, were synthesized by the Fmoc solid phase peptide synthesis. Then, precursor peptides were radioiodinated via electrophilic destannylation, and they were deprotected to obtain F(
Bu)L-OH, were synthesized by the Fmoc solid phase peptide synthesis. Then, precursor peptides were radioiodinated via electrophilic destannylation, and they were deprotected to obtain F(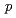 -
- I)KCCYSL and F(
I)KCCYSL and F(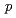 -
- I)GSGKCCYSL in radiochemical yield 15% and 17%, respectively.
I)GSGKCCYSL in radiochemical yield 15% and 17%, respectively.  assays of the radioiodinated peptides for HER2 and stability in serum are being undertaken.
assays of the radioiodinated peptides for HER2 and stability in serum are being undertaken.