Dissolution behavior of calcium uranate under oxidizing and reducing conditions
酸化・還元条件下におけるカルシウムウラネートの溶解挙動
加藤 雄斗*; 佐々木 隆之*; 頓名 龍太郎*; 小林 大志*; 岡本 芳浩 

Kato, Yuto*; Sasaki, Takayuki*; Tonna, Ryutaro*; Kobayashi, Taishi*; Okamoto, Yoshihiro
To assess the chemical stability of CaUO in different aqueous environments, static immersion tests were performed under various redox conditions and carbonate ion concentrations. The dissolution mechanism of CaUO
in different aqueous environments, static immersion tests were performed under various redox conditions and carbonate ion concentrations. The dissolution mechanism of CaUO was examined using data from solid and liquid analyses, along with chemical thermodynamic calculations. Under reducing conditions and without carbonate, U(VI) in CaUO
was examined using data from solid and liquid analyses, along with chemical thermodynamic calculations. Under reducing conditions and without carbonate, U(VI) in CaUO was reduced to U(V) and the mineral was converted into non-stoichiometric CaUO
was reduced to U(V) and the mineral was converted into non-stoichiometric CaUO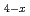 . The dissolved uranium was then further reduced to U(IV) in the aqueous media, forming UO
. The dissolved uranium was then further reduced to U(IV) in the aqueous media, forming UO (am), which controlled the U solubility. Under oxidizing conditions and in the absence of carbonate, dissolved uranium formed metaschoepite ((UO
(am), which controlled the U solubility. Under oxidizing conditions and in the absence of carbonate, dissolved uranium formed metaschoepite ((UO )2H
)2H O(cr)) at pH
O(cr)) at pH 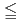 7 and sodium diuranate (Na
7 and sodium diuranate (Na U
U O
O H
H O(cr)) at pH
O(cr)) at pH  7, which controlled uranium solubility. In oxidizing conditions with carbonate present, the apparent solubility of U was lower than predicted by the solid-phase solubility calculations. The concentration of U was constrained to levels similar to that of calcium when the calcium concentration reached saturation with CaCO
7, which controlled uranium solubility. In oxidizing conditions with carbonate present, the apparent solubility of U was lower than predicted by the solid-phase solubility calculations. The concentration of U was constrained to levels similar to that of calcium when the calcium concentration reached saturation with CaCO . Additionally, the dissolution of calcium from CaUO
. Additionally, the dissolution of calcium from CaUO influenced uranium dissolution.
influenced uranium dissolution.