Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hoshino, Tsuyoshi; Tsuchiya, Kunihiko; Hayashi, Kimio; Nakamura, Mutsumi*; Terunuma, Hitoshi*; Tatenuma, Katsuyoshi*
Journal of Nuclear Materials, 386-388, p.1107 - 1110, 2009/04
Times Cited Count:9 Percentile:51.65(Materials Science, Multidisciplinary)no abstracts in English
Sato, Fuminori; Terunuma, Hitoshi*; Arai, Osamu*; Myochin, Munetaka
Nihon Genshiryoku Gakkai Wabun Rombunshi, 8(1), p.83 - 94, 2009/03
Oxide conversion using water vapor and boron oxide (B O
O ) was studied to treat salt waste from dry reprocessing. Parameter tests to CsCl and NaCl-2CsCl salt were performed and fundamental data such as conversion rate, etc. were acquired. To understand the process behavior, a reaction analysis based on thermodynamic equilibrium calculation considering salt (NaCl, CsCl), oxide (Na
) was studied to treat salt waste from dry reprocessing. Parameter tests to CsCl and NaCl-2CsCl salt were performed and fundamental data such as conversion rate, etc. were acquired. To understand the process behavior, a reaction analysis based on thermodynamic equilibrium calculation considering salt (NaCl, CsCl), oxide (Na O, Cs
O, Cs O, B
O, B O
O ) and gas (H
) and gas (H O, Ar, HCl, NaCl, CsCl) phase was performed. The validity of analysis was confirmed by comparison with the experiment. Using this result, process condition of the oxide conversion (ex. temperature, added amount of H
O, Ar, HCl, NaCl, CsCl) phase was performed. The validity of analysis was confirmed by comparison with the experiment. Using this result, process condition of the oxide conversion (ex. temperature, added amount of H O and B
O and B O
O , etc.) was discussed.
, etc.) was discussed.
 W/
W/ Re generators
Re generatorsMatsuoka, Hiromitsu; Hashimoto, Kazuyuki; Hishinuma, Yukio*; Ishikawa, Koji*; Terunuma, Hitoshi*; Tatenuma, Katsuyoshi*; Uchida, Shoji*
Journal of Nuclear and Radiochemical Sciences, 6(3), p.189 - 191, 2005/12
Applicability of Mo adsorbent PZC(Poly Zirconium Compound) for  W/
W/ Re generator was investigated. Long term stability of adsorption of
Re generator was investigated. Long term stability of adsorption of  W to the PZC column, elution of
W to the PZC column, elution of  Re from PZC column, desorption of
Re from PZC column, desorption of 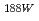 from PZC column, and labeling of Hydroxyethylidene Diphosphonic Acid(HEDP) and Mercaptoacetyltriglycine(MAG3) with
from PZC column, and labeling of Hydroxyethylidene Diphosphonic Acid(HEDP) and Mercaptoacetyltriglycine(MAG3) with  Re eluted from PZC column were tested. The PZC generator gave reproducible
Re eluted from PZC column were tested. The PZC generator gave reproducible  Re elution yields with low
Re elution yields with low  W parent breakthrough for a long period of time(about 5 months), that is the
W parent breakthrough for a long period of time(about 5 months), that is the  W/
W/ Re generator using PZC has a potential for practical use.
Re generator using PZC has a potential for practical use.
Sato, Fuminori; Myochin, Munetaka; Terunuma, Hitoshi*; Arai, Osamu*
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 4 Pages, 2005/10
Oxide conversion of molten salt wastes in pyrochemical reprocessing was studied as a pre-treatment of vitrification. A new method using boron oxide and water vapor was suggested from consideration of a conventional method using boric acid (H3BO3) by chemical equilibrium calculation. Applying the new method for NaCl-CsCl salt in a small-scale experiment, it was confirmed that the most of the salt was converted to oxide and the amount of the oxide wastes after the treatment could be reduced by 20% compared with the conventional method.
Terunuma, Hitoshi*; Arai, Osamu*
JNC TJ8400 2004-037, 73 Pages, 2004/02
In order to convert a salt waste from dry reprocessing to oxide for vitrification, a basic examination about the conversion reaction of chloride was performed. The salt waste simulator (chloride salt) was reacted with boron oxide and water vapor at 1023K-1123K, and converted to oxide with occurring of hydrogen chloride as by-product. the hydrogen chloride generated in this reaction was stabilized by hydrogen chloride stabilizer
Sato, Fuminori; Myochin, Munetaka; Terunuma, Hitoshi*; Arai, Osamu*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 3(3), p.307 - 311, 2004/00
Out-of-pile experiments on the release of fission products (FPs) under transient heating conditions were carried out for mixed oxide fuels. The fuels used in the experiments, the plutonium content of which was 30wt%, were irradiated up to 65 GWd/t in the experimental fast reactor JOYO. The experiments consisted of two runs, FP-1 and FP-2. In FP-1, the fuel sample was first heated to 2,000 C and then up to 3,000
C and then up to 3,000 C. The holding time was 30 min at each temperature. In FP-2, the terminal temperatures were 1,500
C. The holding time was 30 min at each temperature. In FP-2, the terminal temperatures were 1,500 C and 2,500
C and 2,500 C, and the holding time was 30 min in the same manner. The release of Cs, a volatile FP, was detected as soon as the fuel sample was heated up. The release rate, after peaking in several minutes, decreased gradually via a diffusion process in the fuel matrix. The "gross" diffusion coefficient agreed well with the data reported in other experiments using LWR fuels. The release fractions were identical in both experiments, namely Cs 100%, Sb 100%, Ru 10%, Ce 0% and Eu 0%.
C, and the holding time was 30 min in the same manner. The release of Cs, a volatile FP, was detected as soon as the fuel sample was heated up. The release rate, after peaking in several minutes, decreased gradually via a diffusion process in the fuel matrix. The "gross" diffusion coefficient agreed well with the data reported in other experiments using LWR fuels. The release fractions were identical in both experiments, namely Cs 100%, Sb 100%, Ru 10%, Ce 0% and Eu 0%.
Terunuma, Hitoshi*; Arai, Osamu*
JNC TJ8400 2001-056, 101 Pages, 2002/02
Oxidation exchange treatment, chlorine is separated from salt wastes generated from dry reprocessing as a from of hydrogen chlolide by a reaction between salt waste and vapor at high temperature, was investigated for the purpose of adaptation of salt waste to the glassification. According to the last experiments, it has been clarified that the addition of boron oxide was effective to the oxidation exchange treatment of chloride. On the other hand, a phenomenon which chlolide is evaporated by high temperature and effused from the treatment was occurred. Therefore, this phenomenon has been a problem for the practical adaptation. In this study, experiments attach importance to the oxidation exchange of cesium chlolide, the fission products, were performed for the purposes of effective oxidation exchange treatment of chlolide and evaporation reduction of chloride. As a result of this experiment, the evaporation reduction of chlolide was confirmed by reconstructions of equipment such as a vessel for samples and a buffle plate. It has been clarified that nearly 100% of chloride could be separated from the salt waste by oxidation exchange treatment by addition of boron oxide, the oxidation stimulator, to a specimen of cesium chlolide and a mixed specimen of sodium chloride and cesium chloride. Furthermore, some amount of cesium and sodium were remained in the vessel for the treatment and the evaporation of chloride was depressed. Hydrosodalite was applied in order to adsorb and remove hydrogen chloride from the treated specimen, however, the effects of its was unknown and there was a problem that chloride is regenerated by the thermal separation.
Terunuma, Hitoshi*; Arai, Osamu*
JNC TJ8400 2001-004, 96 Pages, 2001/03
For a purpose of glassification processing of the salt waste generated from the fused salt electrolysis method, one of the nuclear fuel reprocessing methods, a processing method to convert chloride to oxide which has an ability of high solubility to the glass was examined. Chloride, the main ingredient of the salt waste, was fused and reacted with water steam. Then the chlorine was separated as a form of hydrogen chloride. The examination was proceeded by an equipment consists of the following compositions. Water steam is generated from the moisture gas generator by the vapor pressure method. The steam is passed through the processing container where the imitation salt is fused and oxidized. Hydrogen chloride generated from the oxidation conversion of chloride is recovered in wet process by the gas scrubber. This examination was implemented under different conditions of imitation salt, processing temperature, water addition amount and presence of boron oxide addition for the stimulation of the oxidation. Volatilized salt steam switched over from the fused salt to the exhaust system, and it caused the plumbing blockade. This phenomenon exerted a big influence on the examination running. By adding boron oxide, percentage of the oxidation conversion increased from a few % to 50%. The oxide generated from processed salt by conversion could be confirmed. It showed that the addition of boron oxide is effective for oxidation conversion of chloride. However, boron oxide was added as the same amount as a commensurate mole of chloride in the imitation salt, and it causes to decline the processing quantity and to increase the amount of the waste evolution. An investigation of the optimal quantity of boron oxide addition will be a next problem to be solved in the future.
; ; Miyazaki, Hitoshi; ; Tanimoto, Kenichi; Terunuma, Seiichi
PNC TN9420 95-011, 13 Pages, 1994/10
None
; Miyazaki, Hitoshi; ; Tanimoto, Kenichi; Terunuma, Seiichi
PNC TN9420 94-010, 103 Pages, 1994/04
None
 W/
W/ Re generator
Re generatorHashimoto, Kazuyuki; Hishinuma, Yukio*; Ishikawa, Koji*; Terunuma, Hitoshi*; Tatenuma, Katsuyoshi*; Uchida, Shoji*; Matsuoka, Hiromitsu
no journal, ,
Applicability of Mo adsorbent PZC(Poly Zirconium Compound) for  W/
W/ Re generator was investigated. Up to the present, long term stability of adsorption of
Re generator was investigated. Up to the present, long term stability of adsorption of  W to the PZC column, elution of
W to the PZC column, elution of  Re from PZC column, desorption of
Re from PZC column, desorption of  W from PZC column, and labeling of Hydroxyethylidene Diphosphonic Acid (HEDP) and Mercaptoacetyltriglycine (MAG3) with
W from PZC column, and labeling of Hydroxyethylidene Diphosphonic Acid (HEDP) and Mercaptoacetyltriglycine (MAG3) with  Re eluted from PZC column were tested and the results were compared with that of
Re eluted from PZC column were tested and the results were compared with that of  Re eluted from alumina column.
Re eluted from alumina column.
Terunuma, Hitoshi*; Nakamura, Mutsumi*; Tatenuma, Katsuyoshi*; Tsuchiya, Kunihiko; Hoshino, Tsuyoshi; Hayashi, Kimio
no journal, ,
no abstracts in English
 powder) in mechanical treatment cell at Tokai Reprocessing Plant
powder) in mechanical treatment cell at Tokai Reprocessing PlantFuruuchi, Yuta; Sato, Shinji; Yatabe, Hitoshi; Yokota, Satoru; Yamada, Takashi; Yahagi, Fumio; Terunuma, Hirotaka; Tokoro, Takeshi; Takahashi, Akihiro; Iijima, Shizuka; et al.
no journal, ,
Clean-up activity of spent fuel powder (UO powder) in mechanical treatment cell was performed for the purpose of the preparation of decommissioning at TRP. For the clean-up activity, we selected an inexpensive vacuum cleaner and made tools, that was improved taking into account of use by means of a crane or a manipulator in the high dose cell, and applied it after a mock-up test. We report our experience and knowledge provided through this clean-up activity.
powder) in mechanical treatment cell was performed for the purpose of the preparation of decommissioning at TRP. For the clean-up activity, we selected an inexpensive vacuum cleaner and made tools, that was improved taking into account of use by means of a crane or a manipulator in the high dose cell, and applied it after a mock-up test. We report our experience and knowledge provided through this clean-up activity.