Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ogawa, Yusuke*; Suzuki, Satoru*; Taniguchi, Naoki; Kawasaki, Manabu*; Suzuki, Hiroyuki*; Takahashi, Rieko*
Materials and Corrosion, 72(1-2), p.52 - 66, 2021/01
Times Cited Count:2 Percentile:12.73(Materials Science, Multidisciplinary)Cast steel is one of the promising alternative to forged steel that is the current reference material for carbon steel overpack. In this study, the full-scale cast steel overpack was produced experimentally and the distribution of casting defects were investigated. The corrosion test regarding corrosion rate and stress corrosion cracking (SCC) susceptibility were also conducted using samples taken from the full-scale cast steel overpack and the corrosion resistance of cast steel was compared with that of forged steel. From above two corrosion tests, it can be said that the corrosion resistance of cast steel is mostly the same as that of forged steel.
Taniguchi, Naoki; Suzuki, Hiroyuki; Kawasaki, Manabu; Naito, Morimasa; Kobayashi, Masato*; Takahashi, Rieko*; Asano, Hidekazu*
Corrosion Engineering, Science and Technology, 46(2), p.117 - 123, 2011/04
Times Cited Count:9 Percentile:47.01(Materials Science, Multidisciplinary)Carbon steel has been selected as one of the candidate materials for overpack for geological disposal of high-level radioactive waste in Japan. Corrosion of carbon steel is divided into two types; general corrosion and localized corrosion. In this study, propagation behaviors of general and localized corrosions (pitting corrosion and crevice corrosion) were investigated by immersion tests of carbon steel under aerobic condition. The results of the immersion tests showed that the growth rate of corrosion was strongly dependent on the environmental condition and steel type, but the upper limit of pitting factor (the ratio of the maximum corrosion depth and the average corrosion depth) was approximately determined by only average corrosion depth. Based on these experimental data and literature data, an empirical model that predicts the maximum corrosion depth of an overpack from average corrosion depth was developed by applying the extreme value statistical analysis using the Gumbel distribution function.
Taniguchi, Naoki; Suzuki, Hiroyuki*; Naito, Morimasa
JAEA-Research 2009-068, 31 Pages, 2010/03
Corrosion of metal is an interaction between the material and the environment, so that the corrosion behavior of carbon steel overpack might be affected by not only the environmental factors but also the material factors. In this study, the effect of general impurities in carbon steel such as C, Si, Mn, P and S on the electrochemical behavior and the corrosion rate were studied using carbonate aqueous solution and synthetic seawater. The experimental results were summarized as follows; (1) The effect of the impurities on the critical passivation current density, 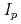 and the passive current density,
and the passive current density, 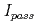 were small in 0.01M carbonate aqueous solution at pH10. (2) Breakdown of passive film and increase in anodic dissolution were observed in the tests for high Si condition of 0.73% and 0.97%. (3) In buffer material saturated with 0.01M carbonate aqueous solution, no passivation was observed and the effect of impurities on the anodic polarization behavior was small. (4) The corrosion rate of carbon steel in seawater was increased with the concentration of impurities. Among the impurity elements, the effects of P and Mn were relatively large. (5) It was inferred that the increase in corrosion rates in synthetic seawater by the addition of Si, Mn and P was promoted by the activation of hydrogen evolution reaction as a cathodic reaction.
were small in 0.01M carbonate aqueous solution at pH10. (2) Breakdown of passive film and increase in anodic dissolution were observed in the tests for high Si condition of 0.73% and 0.97%. (3) In buffer material saturated with 0.01M carbonate aqueous solution, no passivation was observed and the effect of impurities on the anodic polarization behavior was small. (4) The corrosion rate of carbon steel in seawater was increased with the concentration of impurities. Among the impurity elements, the effects of P and Mn were relatively large. (5) It was inferred that the increase in corrosion rates in synthetic seawater by the addition of Si, Mn and P was promoted by the activation of hydrogen evolution reaction as a cathodic reaction.
Taniguchi, Naoki; Suzuki, Hiroyuki*; Naito, Morimasa
JAEA-Research 2009-066, 18 Pages, 2010/03
It has been known that carbon steel can be passivated in high pH environment and sometimes be attacked by localized corrosion such as pitting corrosion, crevice corrosion. If carbon steel overpack is attacked by localized corrosion, it is possible that the overpack may be penetrated in a short term, since the propagation rate of localized corrosion is larger than that of general corrosion in general. It has been known that the pitting corrosion and crevice corrosion are initiated in the presence of aggressive species for passive film represented by chloride ion. In repository environment, it is possible that the pH in groundwater containing chloride ion such as seawater type groundwater is raised by a contact with the cement material in concrete structures constructed around the engineered barrier system and then pitting corrosion or crevice corrosion is caused on the carbon steel overpacks. In this study, the propagation behavior of pitting corrosion and crevice corrosion was examined by immersion tests under air equilibrium condition using artificial groundwater at Horonobe as an example of seawater-type groundwater. As the results, the pitting factor (ratio of the maximum corrosion depth and average corrosion depth) were within the literature data obtained in neutral and alkaline water and in various natural water environments. The maximum corrosion depth of carbon steel overpack was predicted by extreme value statistical analysis of the experimental data, and it was confirmed that the predicted corrosion depths were not over the values calculated from current empirical models for propagation of pitting corrosion and crevice corrosion in every cases.
Taniguchi, Naoki; Suzuki, Hiroyuki*; Nakanishi, Tomoaki*; Nakayama, Takenori*; Masugata, Tsuyoshi*; Tateishi, Tsuyoshi*
Zairyo To Kankyo, 56(12), p.576 - 584, 2007/12
The long term hydrogen absorption behavior and the possibility of hydrogen embrittlement were studied for titanium overpack for high level radioactive waste disposal. The results of galvanostatic cathodic polarization tests showed that as the cathodic current density is lowered, the amount of absorbed hydrogen for a constant cathodic charge was increased as well as hydrogen permeated into inside of titanium. The hydrogen absorption ratio for a cathodic current density equivalent to the corrosion rate under anaerobic condition was estimated to nearly 100 percent, and the amount of absorbed hydrogen for 1000 years was evaluated to be 400 ppm. The mechanical property of titanium containing hydrogen depended on not only hydrogen concentration but also hydrogen distribution type. The more hydrogen distribution is uniform, the degree of embrittlement was larger. It was expected that the rupture of titanium overpack with 6 mm thickness would be initiated if the crack size in titanium is over about 2-3 mm under the stress corresponds to yield strength.
Suzuki, Hiroyuki*; Taniguchi, Naoki
Zairyo To Kankyo, 55(11), p.485 - 494, 2006/11
Titanium is one of the candidate materials for overpacks as a high corrosion resistance metal. Hydrogen embrittlement is a main cause of the damage of long term integrity of titanium overpack. It is not well known about the corrosion resistance and hydrogen absorption behavior of titanium under anaerobic condition. In this study, the completely sealed ampoule test and the immersion test of titanium was carried out in aqueous solution and bentonite in order to obtain reliable data about the hydrogen generation rate and the ratio of hydrogen absorption in titanium. As the results of the tests with changing the environmental factors, obvious higher corrosion rates were observed at high carbonate (1M) and high pH(pH13) conditions due to the increase in the anodic reaction rate. In other conditions, corrosion rate of titanium were estimated to be in the order of 10
 10
10
 m/y. Almost all (
m/y. Almost all ( 98%) of the hydrogen generated by corrosion was absorbed into titanium. Assuming that the time evolution of the hydrogen content in titanium follows linear law to make conservative assessment, the absorbed hydrogen content was estimated to be of 400
98%) of the hydrogen generated by corrosion was absorbed into titanium. Assuming that the time evolution of the hydrogen content in titanium follows linear law to make conservative assessment, the absorbed hydrogen content was estimated to be of 400  500ppm in 1000 years.
500ppm in 1000 years.
 SR
SRShimomura, Koichiro*; Kadono, Ryosuke*; Nishiyama, Kusuo*; Watanabe, Isao*; Suzuki, Takao*; Pratt, F.*; Oishi, Kazuki; Mizuta, Masashi*; Saito, Mineo*; Chow, K. H.*; et al.
Physica B; Condensed Matter, 376-377, p.444 - 446, 2006/04
Times Cited Count:1 Percentile:6.64(Physics, Condensed Matter)Hamamoto, Shimpei; Iigaki, Kazuhiko; Shimizu, Atsushi; Sawahata, Hiroaki; Kondo, Makoto; Oyama, Sunao; Kawano, Shuichi; Kobayashi, Shoichi; Kawamoto, Taiki; Suzuki, Hisashi; et al.
JAEA-Technology 2006-030, 58 Pages, 2006/03
During normal operation of High Temperature engineering Test Reactor (HTTR) in Japan Atomic Energy Agency (JAEA), the reactivity is controlled by the Control Rods (CRs) system which consists of 32 CRs (16 pairs) and 16 Control Rod Drive Mechanisms (CRDMs). The CR system is located in stand-pipes accompanied by the Reserved Shutdown System (RSS). In the unlikely event that the CRs fail to be inserted, the RSS is provided to insert B C/C pellets into the core. The RSS shall be designed so that the reactor should be held subcriticality from any operation condition by dropping in the pellets. The RSS consists of B
C/C pellets into the core. The RSS shall be designed so that the reactor should be held subcriticality from any operation condition by dropping in the pellets. The RSS consists of B C/C pellets, hoppers which contain the pellets, electric plug, driving mechanisms, guide tubes and so on. In accidents when the CRs cannot be inserted, an electric plug is pulled out by a motor and the absorber pellets fall into the core by gravity. A trouble, malfunction of one RSS out of sixteen, occurred during a series of the pre-start up checks of HTTR on February 21, 2005. We investigated the cause of the RSS trouble and took countermeasures to prevent the issue. As the result of investigation, the cause of the trouble was attributed to the following reason: In the motor inside, The Oil of grease of the multiplying gear flowed down from a gap of the oil seal which has been deformed and was mixed with abrasion powder of brake disk. Therefore the adhesive mixture prevented a motor from rotating.
C/C pellets, hoppers which contain the pellets, electric plug, driving mechanisms, guide tubes and so on. In accidents when the CRs cannot be inserted, an electric plug is pulled out by a motor and the absorber pellets fall into the core by gravity. A trouble, malfunction of one RSS out of sixteen, occurred during a series of the pre-start up checks of HTTR on February 21, 2005. We investigated the cause of the RSS trouble and took countermeasures to prevent the issue. As the result of investigation, the cause of the trouble was attributed to the following reason: In the motor inside, The Oil of grease of the multiplying gear flowed down from a gap of the oil seal which has been deformed and was mixed with abrasion powder of brake disk. Therefore the adhesive mixture prevented a motor from rotating.
Suzuki, Hiroyuki*; Taniguchi, Naoki; Kawakami, Susumu
JNC TN8400 2005-003, 76 Pages, 2005/03
Titanium is one of the candidate materials for overpacks as a high corrosion resistance metal.At the initial stage of repository,oxidizing condition will be given around the overpack because oxygen will be brought from the ground. The oxygen will be consumed by the reaction with impurities in buffer material or corrosion of overpack, and reducing condition will be achieved around the overpack. With the changing of redox condition, the water reduction becomes to dominate the cathodic reaction accompanying hydrogen generation. Crevice corrosion and hydrogen embrittlement are main causes of the damage of long term integrity of titanium overpack.However, it is not known about the corrosion resistance and hydrogen absorption behavior of titanium under reduction condition. In this study, the completely sealed ampoule test and the immersion test of titanium in aqueous solution and bentonite was carried out. In order to obtain reliable data about the hydrogen generation rate and the ratio of hydrogen absorption in titanium.From the result of 3 years immersion tests, corrosion rate of titanium were estimated to be in the order of 10 - 10
- 10 micro m / y in the aqueous solution, and 10
micro m / y in the aqueous solution, and 10 - 10
- 10 micro m / y in bentonaite.This value is almost the same as the last report.Almost all the hydrogen generated by corrosion was absorbed in titanium in the immersion tests in completely sealed ampoule.In the examination that changed each parameter, it was suggested that the amount of the hydrogen absorption become 2 - 3 times in 1M HCO
micro m / y in bentonaite.This value is almost the same as the last report.Almost all the hydrogen generated by corrosion was absorbed in titanium in the immersion tests in completely sealed ampoule.In the examination that changed each parameter, it was suggested that the amount of the hydrogen absorption become 2 - 3 times in 1M HCO
 and pH13.
and pH13.
Suzuki, Hiroyuki*; Taniguchi, Naoki; Kawakami, Susumu
JNC TN8400 2003-042, 34 Pages, 2004/03
Titanium is one of the candidate materials for overpacks as a high corrosion resistance metal. Crevice corrosion and hydrogen embrittlement are main causes of the damage of long term integrity of titanium overpack. At the initial stage of repository, oxidizing condition will be given around the overpack because oxygen will be brought from the ground. The oxygen will be consumed by the reaction with impurities in buffer material or corrosion of overpack, and reducing condition will be achieved around the overpack. With the changing of redox condition, the H2O reduction becomes to dominate the cathodic reaction accompanying hydrogen generation. However, it is not known about the corrosion resistance and hydrogen absorption behavior of titanium under reduction condition. In this study, the immersion test of titanium in aqueous solution was carried out. Based on the SIMS analysis, the hydrogen concentration distribution and the oxide film growth behavior were investigated. From the result of immersion tests for 2 years, corrosion rate of titanium were estimated to be in the order of 10-2 - 10-1 micro meter / year in the aqueous solution.
*; Taniguchi, Naoki; Kawakami, Susumu
JNC TN8400 2003-003, 139 Pages, 2003/03
Titanium is one of the candidate materials for overpacks as a high corrosion resistance metal. Crevice corrosion and hydrogen embrittlement are main causes of the damage of long term integrity of titanium overpack. At the initial stage of repository, oxidizing condition will be given around the overpack because oxygen will be brought from the ground. The oxygen will be consumed by the reaction with impurities in buffer material or corrosion of overpack, and reducing condition will be achieved around the overpack. With the changing of redox condition, the H O reduction becomes to dominate the cathodic reaction accompanying hydrogen generation. However, it is not known about the corrosion resistance and hydrogen absorption behavior of titanium under reduction condition. In this study, the immersion test of titanium in aqueous solution and bentonite was carried out. Based on the SIMS analysis, the hydrogen concentration distribution and the oxide film growth behavior were investigated. In order to obtain reliable data about the hydrogen generation rate and the ratio of hydrogen absorption in titanium, the immersion test was carried out in completely sealed ampoule. In addition, galvanostatic acceleration tests were examined to estimate hydrogen absorption ratio after a long time. From the result of short term (
O reduction becomes to dominate the cathodic reaction accompanying hydrogen generation. However, it is not known about the corrosion resistance and hydrogen absorption behavior of titanium under reduction condition. In this study, the immersion test of titanium in aqueous solution and bentonite was carried out. Based on the SIMS analysis, the hydrogen concentration distribution and the oxide film growth behavior were investigated. In order to obtain reliable data about the hydrogen generation rate and the ratio of hydrogen absorption in titanium, the immersion test was carried out in completely sealed ampoule. In addition, galvanostatic acceleration tests were examined to estimate hydrogen absorption ratio after a long time. From the result of short term ( 1 year) immersion tests, corrosion rate of titanium were estimated to be in the order of 10
1 year) immersion tests, corrosion rate of titanium were estimated to be in the order of 10
 10
10
 m/y in the aqueous solution, and 10
m/y in the aqueous solution, and 10
 10
10
 m/y in bentonaite. Almost all the hydrogen generated by corrosion was absorbed in titanium in the immersion tests in completely sealed ampoule for 90days. From the results of electrochemical acceleration tests by galvanostatic method, it was observed that the hydrogen absorption ratio became large with decreasing supplied current density. Based on the acceleration test, we estimated hydrogen absorption ratio of titanium after 1000 years. It was implied that several ...
m/y in bentonaite. Almost all the hydrogen generated by corrosion was absorbed in titanium in the immersion tests in completely sealed ampoule for 90days. From the results of electrochemical acceleration tests by galvanostatic method, it was observed that the hydrogen absorption ratio became large with decreasing supplied current density. Based on the acceleration test, we estimated hydrogen absorption ratio of titanium after 1000 years. It was implied that several ...
Kukita, Yutaka; Nakamura, Hideo; *; *; *; Anoda, Yoshinari; Kumamaru, Hiroshige; Suzuki, Mitsuhiro; ; Yonomoto, Taisuke; et al.
JAERI-M 91-040, 122 Pages, 1991/03
no abstracts in English
Taniguchi, Naoki; Suzuki, Hiroyuki*; Yui, Mikazu; Nakanishi, Tomoaki*; Nakayama, Takenori*; Masugata, Tsuyoshi*; Tateishi, Tsuyoshi*
no journal, ,
Titanium (including titanium alloy) is one of the candidate materials of overpacks for geological disposal of high-level radioactive waste, and required long term integrity against the groundwater for more than 1000 years. As the corrosion of titanium occurs, hydrogen is generated since the deep underground environment is originally low oxygen concentration condition. There is a possibility that the titanium overpack will be attackd by the hydrogen embrittlement due to long term hydrogen absorption. In this study, the amount of hydrogen and the possibility of embrittlement were investigated based on the experimental data on the corrosion rate, hydrogen absorption behavior, mechanical proparty of titanium containing hydrogen.
Taniguchi, Naoki; Suzuki, Hiroyuki*; Naito, Morimasa
no journal, ,
no abstracts in English
Suzuki, Koichi*; Yoshimura, Kimitaka*; Sugita, Yutaka; Ando, Makoto*; Azuma, Hiroyuki*
no journal, ,
One of the benefits of geophysical exploration is that extensive and continuous surveys are possible at low cost, however, the obtained physical properties are chiefly velocity of elastic wave and electrical resistivity. Therefore, it is very important that the techniques can convert geophysical data into quantitative information on the geological environment required for engineering technology. In this study, we have applied an approach that provides estimates of porosity, clay content, and equivalent NaCl concentration based on a combined interpretation of geophysical survey data utilizing an expanded bimodal mixture model. The study used seismic velocity and electrical conductivity data obtained from geophysical logging at two soft sedimentary rock sites, in the muddy or tuffaceous sandstone and mudstone at Yokosuka, and in diatomaceous mudstone and fine sandstone at Horonobe, Japan. The geological information for these sites was successfully estimated.
Taniguchi, Naoki; Suzuki, Hiroyuki; Kawasaki, Manabu; Watanabe, Masatoshi; Tateishi, Tsuyoshi*; Sazarashi, Masami
no journal, ,
Immersion tests of carbon steel were carried out in bentonite saturated with synthetic seawater between 30-160  C. The corrosion depth was measured by weight loss of the specimens. The results showed that the initial corrosion rates increased with increasing temperature, and that the most protective corrosion product film was formed at 80-120
C. The corrosion depth was measured by weight loss of the specimens. The results showed that the initial corrosion rates increased with increasing temperature, and that the most protective corrosion product film was formed at 80-120  C. In order to evaluate the possibility of hydrogen embrittlement, the content of absorbed hydrogen (diffusive hydrogen) in specimens were analyzed. The results showed a tendency of decreasing the content of absorbed hydrogen with increasing temperature.
C. In order to evaluate the possibility of hydrogen embrittlement, the content of absorbed hydrogen (diffusive hydrogen) in specimens were analyzed. The results showed a tendency of decreasing the content of absorbed hydrogen with increasing temperature.
Ito, Rintaro; Suzuki, Kenta; Horiuchi, Kazunori; Kawabata, Kuniaki; Kawatsuma, Shinji; Suzuki, Hiroyuki*; Dekura, Toshinori*
no journal, ,
This paper, describe the development of the operation training function for repair work of leakage part of reactor primary containment vessel by remotely operated manipulator using VR. Remote operation of the manipulator with limited feedback information is difficult and therefore prior operation proficiency training is important to complete such difficult task safely. Therefore, we are developing an operation training function that enables the operator not only to perform operation training, but also to confirm the operation of the remote control device actually operated by himself in the actual size and three-dimensionally in the VR space. In this paper, we reported current status of the development and recent improvements for operation training function.
Kikuchi, Ryosuke*; Fujimura, Tatsuya*; Sato, Tsutomu*; Otake, Tsubasa*; Otomo, Yoko*; Goto, Takahiro*; Suzuki, Satoru*; Taniguchi, Naoki; Suzuki, Hiroyuki*
no journal, ,
Suzuki, Hiroyuki*; Kitayama, Ayami; Mitsui, Seiichiro; Taniguchi, Naoki
no journal, ,
The environmental conditions around carbon steel overpacks in geological disposal are expected to be diverse cases depending on geological environment of the repository and may also vary depending on changes in conditions after repository closure. In this study, with the aim of understanding corrosion behavior under such environmental conditions, immersion tests with different conditions were conducted in bentonite/silica sando mixture assuming a buffer material to be constructed around the overpack under a wide range of conditions, including temperature and water quality beyond the assumed range of conventional environmental conditions, and the effects on corrosion behavior were evaluated.
Taniguchi, Naoki; Kitayama, Ayami; Kawasaki, Manabu*; Nakayama, Masashi; Ono, Hirokazu; Mitsui, Seiichiro; Suzuki, Hiroyuki*
no journal, ,
no abstracts in English