Crystallization and preliminary X-ray diffraction studies of the catalytic domain of a novel chitinase, a member of GH family 23, from the moderately thermophilic bacterium 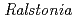 sp. A-471
sp. A-471
好熱菌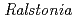 sp. A-471由来新規キチナーゼ触媒ドメインの結晶化及びX線回折研究
sp. A-471由来新規キチナーゼ触媒ドメインの結晶化及びX線回折研究
岡崎 伸生; 有森 貴夫; 中澤 昌美*; 宮武 和孝*; 上田 光宏*; 玉田 太郎
Okazaki, Nobuo; Arimori, Takao; Nakazawa, Masami*; Miyatake, Kazutaka*; Ueda, Mitsuhiro*; Tamada, Taro
Chitinase from the moderately thermophilic bacterium 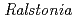 sp. A-471 (Ra-ChiC) is divided into two domains: a chitin-binding domain (residues 36-80) and a catalytic domain (residues 103-252). Although the catalytic domain of Ra-ChiC has homology to goose-type lysozyme, Ra-ChiC does not show lysozyme activity but does show chitinase activity. The catalytic domain with part of an interdomain loop (Ra-ChiC
sp. A-471 (Ra-ChiC) is divided into two domains: a chitin-binding domain (residues 36-80) and a catalytic domain (residues 103-252). Although the catalytic domain of Ra-ChiC has homology to goose-type lysozyme, Ra-ChiC does not show lysozyme activity but does show chitinase activity. The catalytic domain with part of an interdomain loop (Ra-ChiC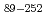 ) was crystallized under several different conditions using polyethylene glycol as a precipitant. The crystals diffracted to 1.85
) was crystallized under several different conditions using polyethylene glycol as a precipitant. The crystals diffracted to 1.85  resolution and belonged to space group
resolution and belonged to space group  6
6 22 or
22 or  6
6 22, with unit-cell parameters
22, with unit-cell parameters  =
=  = 100,
= 100,  = 243
= 243  . The calculated Matthews coefficient was approximately 3.2, 2.4 or 1.9
. The calculated Matthews coefficient was approximately 3.2, 2.4 or 1.9 
 Da
Da assuming the presence of three, four or five Ra-ChiC
assuming the presence of three, four or five Ra-ChiC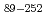 molecules in the asymmetric unit, respectively.
molecules in the asymmetric unit, respectively.