Synthesis and in vivo evaluation of radiohalogen-labeled antitumor cyclic peptides
放射性ハロゲン標識抗腫瘍環状ペプチドの合成とin vivo評価
山田 圭一*; 渡邉 茂樹; 大島 康宏; 花岡 宏史*; 津久井 匠隆*; 高野 智香子*; 山口 藍子*; 奥 浩之*; 石岡 典子
Yamada, Keiichi*; Watanabe, Shigeki; Ohshima, Yasuhiro; Hanaoka, Hirofumi*; Tsukui, Narutaka*; Takano, Chikako*; Yamaguchi, Aiko*; Oku, Hiroyuki*; Ishioka, Noriko
Sansalvamide A (SA) is a marin-derived cyclic depsipeptide that exhibits cytotoxicity against colon cancer cell line. Recently, a series of cyclic pentapeptide analogs of SA in which Hmp ((2S)-2-hydroxy-4-methylpentanoic acid) residue was replaced by amino acids or N-methyl-amino acids were synthesized. We previously reported that the haogenated analog of [MeLeu ]SA, namely SA-Br, SA-I, SA-Cl, exhibited potent cytotoxicity against MDA-MB-231. In the present study, we considered to evaluate in vivo biodistribution, stability and antitumor activity of these halogenated SA peptides in tumor-bearing mice. For this purpose, radiohalogen atoms are most suitable for labeling without changing their primary structure and biological activity. Here, we report the synthesis and in vivo biodistribution of
]SA, namely SA-Br, SA-I, SA-Cl, exhibited potent cytotoxicity against MDA-MB-231. In the present study, we considered to evaluate in vivo biodistribution, stability and antitumor activity of these halogenated SA peptides in tumor-bearing mice. For this purpose, radiohalogen atoms are most suitable for labeling without changing their primary structure and biological activity. Here, we report the synthesis and in vivo biodistribution of 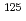 I-labeled SA analog (SA-[
I-labeled SA analog (SA-[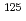 I]).
I]).